The Medical Device Ecosystem and Cybersecurity — Building Capabilities and Advancing Contributions
Medical device cybersecurity is shared responsibility across the medical device ecosystem
- In context of total product lifecycle
- As part of interconnected cyber — physical infrastructure among people, processes, data and information and communication technologies
- Identify and prepare for cyber intrusions, reduce medical device vulnerabilities, mitigate patient impact, enable timely restoration of devices and systems
Applying a best-teams approach (with Dept. of Homeland Security, FDA Medical Device Safety Action Plan, Guidances on Premarket cybersecurity)
Building strategic alliances (with MITRE, MDIC)
Fortifying long-term commitment (Center of Excellence for Digital Health)
FDA Seeking Digital Health Advisor
Primarily serve as expert on digital health technologies during FDA’s review and evaluation of digital health technologies
- Review of first-of-a-kind digital health technology, precedent-setting research
- Create preconditions and incentives informing digital health policy proposals
- Provide advice, consultations and training
- Assist with technology solutions to enhance CDRH internal processes
https://twitter.com/_bakulpatel

Stakeholder Update: FDA’s 2018 Strategic Policy Roadmap
Update on priority areas to advance public health
Reduce the burden of addiction crises
- Address growing epidemic of youth e-cigarette use, including potential new therapies to support cessation Read
- Industry meetings on epidemic rates in youth e-cigarette use Read
Leverage innovation and competition to improve health care and access
- Medical Device Ecosystem and Cybersecurity Read
- Authorization of first direct-to-consumer test for detecting genetic variants for medication metabolism

Approval of Dsuvia and the FDA’s future consideration of new opioids
Dsuvia approval considerations in the context of the overall therapeutic armamentarium
- Sublingual with restricted use in certified medically-supervised health care settings
- Ideally suited for special circumstances where patients not able to swallow oral medication and access to intravenous pain relief is not possible
- Potential uses on the battlefield – Department of Defense (DoD) worked closely with sponsor on the development of opioid
- Very tight restrictions on distribution and use with REMS
- Quickly make regulatory adjustments if problems arise

Growing epidemic of youth e-cigarette use, including potential new therapies to support cessation
Public hearing on Dec. 5, to focus on potential role of drug therapies to support cessation
- Obtain input from across the medical and research fields, the pharmaceutical and tobacco industries, and public health stakeholders
- Approaches to eliminate youth e-cigarette use
- Exploring potential drug therapies to support youth e-cigarette cessation
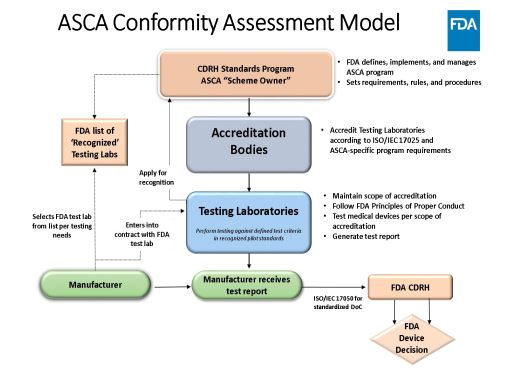
Accreditation Scheme for Conformity Assessment (ASCA)
Piloted under CDRH’s Standards and Conformity Assessment Program
- Enhance predictability of the medical device review process
- Reducing premarket questions on conformity activities
- Conformance declarations
- Increase consistency of submissions and saving FDA resources
Pilot will ensure appropriate interaction between FDA, accreditation bodies, and testing labs

FDA and DoD formalize collaboration to advance medical products in support of American military personnel
New Memorandum of Understanding aligns agency efforts to foster the development and use of safe and effective medical products for members of the U.S. military
- Evaluate how best to foster access to safe and effective medical products
- Expedite review of priority DoD medical products
- Provide technical to aid rapidly develop and manufacture medical products
- Determine opportunities to streamline review and expedite availability
- Authorize emergency uses of medical products to reduce deaths and severity of injuries caused by chemical, biological, radiological or nuclear (CBRN) agents
Image credit: FDA

