Generic Drug Review Dashboard
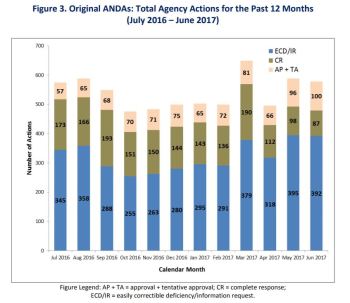
Office of Generic Drugs (OGD) providing update to improve transparency related to Generic Drug User Fee Amendments of 2012 (GDUFA)
- proportion of pending ANDA workload
- proportion of ANDA workload pending response by industry
- ongoing review communications between FDA and industry
Image credit: FDA