
What We Mean When We Talk About Data
By: Robert M. Califf, M.D. and Rachel Sherman, M.D., M.P.H.
Tackling Terminology
EXISTING:
Data : Raw measurements of some thing or process
↓
Information : With critical context about what is being measured
↓
Evidence : With analysis to guide decision-making
EVOLVING
- “real-world data” or “real-world evidence”
- depict what patients and care providers will experience
- help to fundamentally better understand states of disease and health
- however; issues with quality and interpretability of data for regulatory decision making
FDA POSITION: Recognizes challenges and developing effective ways to adapt “real-world data” into processes for creating scientific evidence

MDUFA IV Reauthorization Meeting (DEVICE)
FDA and Industry presented their respective initial proposal packages
Commonality : Around the themes of consistency and predictability in the medical device review program, as well as process improvements that include pre-submission consultations and de novo submissions.
Apparent Differences : In the approaches taken to address the common themes. FDA and Industry anticipate productive discussions on the details of and assumptions underlying the proposal packages.
Overview of FDA Proposals
- Ensuring Consistent, Predictable, and Efficient Review Experience: (i) Quality Management System, (ii) Recruitment, Retention, and Development of Managers and Staff, (iii)Program Reliability, Resiliency, and Transparency,
- Innovative Review Process for Innovative Products : (i) patient preference information in benefit/risk assessment, (ii) link data from health care claims, electronic health records, and registries, (iii) digital health device regulation and smart policy on areas of interoperability, clinical decision support, software modifications, telemedicine, and cybersecurity (iv) device specific guidnaces
PDUFA V Metrics: Programs for Supporting Expedited Development and Enhanced Reviews (DRUG)
Oct 2014 – Sept 2015

Expedited Drug Development and Review, intended to Treat Serious or Life-Threatening Conditions, Potential to address Unmet Need
Approval based on Surrogate Endpoints reasonably likely to Predict Clinical Benefit

Treatment of Rare Diseases/Disorders < 200,000 U.S. patients.
 Earlier FDA Attention and Guidance (Cross-Disciplinary Team w/Senior Mgrs) to Expedite Drugs Development for Serious/Life-Threatening Condition
Earlier FDA Attention and Guidance (Cross-Disciplinary Team w/Senior Mgrs) to Expedite Drugs Development for Serious/Life-Threatening Condition
Independent Assessment of PDUFA V metrics (thus far):
- Enhanced review transparency and communication
- Enhanced predictability of reviews and resolve approvability issues promptly
- Enhanced opportunities for FDA and Sponsor to address issues to increase first cycle approval
- Label Review Good Practics : Providing explanations/rationales for proposed label
- However, Inconsistent availability/communication of information about inspections
Full 2015 metrics anticipated in 2016. Stay Tuned.


VISTOGUARD (uridine triacetate)
ORAL GRANULES
Indication: Emergency treatment of adult and pediatric patients:
- Following a fluorouracil or capecitabine overdose
- Who exhibit early-onset, severe or life-threatening toxicity within 96 hours following the end of fluorouracil or capecitabine administration.
Unmet Need:
- Fluorouracil (taken by infusion) and capecitabine (taken orally) used for decades to treat several types of cancer
- While rare, unintentional overdose can occur
- Need for therapy to potentially save lives following overdose or life-threatening toxicity
Reg Pathway : NDA orphan drug designation, priority review and fast track
Mechanism of Action : After oral administration, uridine triacetate is deacetylated to uridine that competitively inhibits cell damage and cell death caused by fluorouracil.
Efficacy:
- 2 open-label trials, Study 1 (n=60) and Study 2 (n=75)
- Primary measure: Survival at 30 days or chemotherapy resumption
- Overdose : 97% still alive at 30 days
- Toxicity onset : 89% alive at 30 days
- 33 % resumed chemotherapy in less than 30 days.
Safety: Diarrhea, vomiting and nausea.
 KANUMA (sebelipase alfa)
KANUMA (sebelipase alfa)
INJECTION
Indication : Treatment of Lysosomal Acid Lipase (LAL) deficiency.
Unmet Need:
- LAL deficiency is a rare inherited genetic disorder that can lead to serious and life-threatening organ damage, especially when onset begins in infancy
- Need for treatment that may improve their lives and chances of survival
Reg Pathway: BLA, Orphan Drug Designation, Breakthrough Therapy Designation, Priority Review and Rare Pediatric Disease Priority Review Voucher
- Reviews by 2 FDA centers:
- Center for Veterinary Medicine (CVM) for a recombinant DNA (rDNA) construct in chickens genetically engineered to produce recombinant form of human lysosomal acid lipase (rhLAL) protein in egg whites
- The Center for Drug Evaluation and Research (CDER) for efficacy and safety of human therapeutic biologic (Kanuma)
Mechanism of Action: Deficient LAL enzyme activity results in progressive complications due to the lysosomal accumulation of cholesteryl esters and triglycerides in multiple organs. Kanuma binds to cell surface receptors and catalyzes lysosomal hydrolysis of cholesteryl esters and triglycerides
Efficacy:
- Multicenter, open-label, single-arm study (n=9 infants)
- Primary Endpoint: Survival vs untreated historical cohort (n=21)
- Survival beyond 12 mo. of age : 6 vs 0
- Median age of surviving patients: 18.1 mo. (range 12 – 42.2 mo.)
Safety : Diarrhea, vomiting, fever, rhinitis, anemia, cough, headache, constipation, and nausea.
 VONVENDI [von Willebrand factor (Recombinant)]
VONVENDI [von Willebrand factor (Recombinant)]
INTRAVENOUS INJECTION (Lyophilized Powder for Solution)
Indication: On-demand treatment and control of bleeding episodes in adults (age 18 and older) diagnosed with von Willebrand disease (VWD)
Unmet need:
- VWD is most common inherited bleeding disorder, affecting approximately 1 percent of U.S. population
- Severe bleeding from the nose, gums, intestines, muscles and joints. heavy menstrual periods, excessive bleeding after childbirth
- Need for additional therapeutic option for treatment of bleeding episodes
Reg Pathway: BLA, Orphan product designation
Mechanism of Action: Promote hemostasis by mediating platelet adhesion and as a carrier protein for factor VIII, protecting it from rapid proteolysis.
Efficacy:
- Multicenter, open label trial (n=69), with vs. without recombinant factor VIII for on-demand treatment and control of bleeding episode
- Primary Endpoint : Number of subjects with treatment success based on pre-specified 4 point rating scale
- Rating of excellent (96.9%) or good (3.1%)
- Control of bleeding episodes was consistent across all degrees of severity.
Safety: Most common adverse reaction observed was generalized pruritus
 ALECENSA® (alectinib)
ALECENSA® (alectinib)
CAPSULE
Indication: Treatment of anaplastic lymphoma kinase (ALK)-positive,
metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Unmet Need:
- Lung cancer leading cause of cancer death in US
- Estimated 221,200 new diagnoses and 158,040 deaths in 2015
- ALK gene mutations are present in about 5 percent of patients
- Brain is a common place for the disease to spread
- Need for new therapy for patients who no longer respond to Xalkori
Reg Pathway: NDA, accelerated approval, breakthrough therapy designation and priority review status. Also received orphan drug designation,
Mechanism of Action: Tyrosine kinase inhibitor that targets ALK and RET.
Efficacy: Approval based on tumor response rate and duration of response. Confirmatory trial required
- 2 single-arm, multicenter clinical trials (n=87 and 132)
- Major efficacy outcome : Objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumours (RECIST v1.1) by Independent Review Committee (IRC)
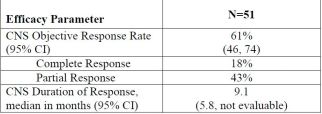
- Additional outcome measures : Duration of response (DOR), CNS ORR, and CNS DOR.
Efficacy in Study 1 and 2

CNS Efficacy
Safety:
- Most common side effects : Fatigue, constipation, swelling (edema) and muscle pain (myalgia).
- Serious side effects: Liver problems, severe or life-threatening inflammation of the lungs, very slow heartbeats and severe muscle problems
- May cause sunburn when patients are exposed to sunlight

Dignitana DigniCap Cooling System
Dignitana Inc., Lund, Sweden
Indication for Use : Reduce the likelihood of chemotherapy-induced alopecia in women with breast cancer.
Unmet Need:
- Hair loss is a common side effect of certain types of chemotherapy, commonly associated with the treatment of breast cancer
- Minimizing or relieving hair loss contributes to improving quality of life and overall health and recovery
Reg Pathway:
- De Novo evaluation
- Regulation Name: Scalp Cooling System to Reduce the Likelihood of
Chemotherapy-Induced Alopecia - Regulatory Classification: Class II
Device Description: Computer-controlled system that circulates cooled liquid to a head-worn cooling cap during chemotherapy treatment. The cooling cap is covered by a second cap made from neoprene, which holds the cooling cap in place and acts as an insulation cover to prevent loss of cooling.
Efficacy:
- Stage I and Stage II breast cancer undergoing chemotherapy (n=122)
- Primary endpoint: Self-assessment of hair loss using standardized photographs
- 66 percent of patients reported losing less than half their hair
Safety: Cold-induced headaches and neck and shoulder discomfort, chills, and pain

XSTAT 30 wound dressing
RevMedX, Inc., Wilsonville, Ore.
Indication for Use: Patients at high risk for immediate, life-threatening, and severe hemorrhagic shock and non-compressible junctional wounds, when definitive care at an emergency care facility cannot be achieved within minutes.
Unmet Need:
- Early control of severe bleeding may prevent shock and may be life-saving.
- 30 to 40 percent of civilian deaths by traumatic injury are the result of hemorrhaging – 33 to 56 percent occur before the patient reaches a hospital.
- Need for dressing to control severe, life-threatening bleeding from wounds in battlefield and civilian trauma settings
Reg Pathway: 510 K, Class II, substantially equivalent to the XSTAT
Device Description:
- Syringe-style applicators containing 92 compressed, cellulose sponges that have an absorbent coating.
- Sponges expand and swell to fill the wound cavity, creating a temporary physical barrier to blood flow.