News and Views: Novel device innovation, Alternative nonclinical testing, Technology Modernization Action Plan, CDRH regulatory science tools, Drug safety priorities, CDER ISTAND program
Novel Device Innovation Despite COVID-19 Challenges
FDA approved, cleared, or authorized (“authorized”) 132 novel medical devices in 2020 (vs. 29 novel devices authorized in 2010)
- Novel devices include those brought to market through PMA, HDE De Novo pathways and only those 510(K) with Breakthrough designation. Also first-of-a-kind devices authorized under EUA authority
- Devices have ‘new’ technology AND address an unmet need, or may be safer or more effective than currently available alternatives
Examples include:
- Game-based digital therapeutic to improve attention function in children with ADHD
- Anterior cruciate ligament (ACL) implant as an alternative to ACL reconstruction to treat ACL tears
- Continuous renal replacement therapy device for a lower weight pediatric population
- Liquid biopsy next-generation sequencing companion diagnostic test
- Cardiac ultrasound software that uses artificial intelligence (AI) for quality diagnostic images
- Automated insulin delivery and monitoring system for use in young pediatric patients
- High density lipoprotein plasma delipidation system to reduce coronary artery atheroma in certain adult patients
- High intensity focused ultrasound (HIFU), magnetic-resonance guided, ablation system for osteoid osteomas
- Several COVID-19 specific devices for diagnosing and testing
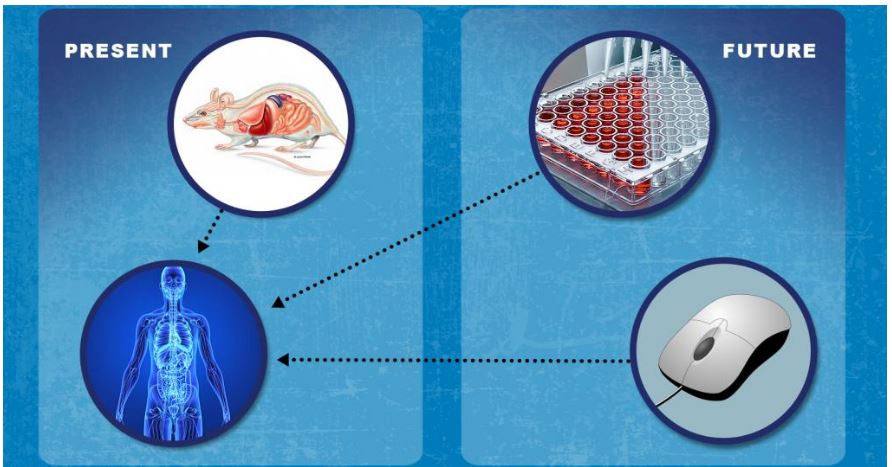
Advancing Alternative Methods for nonclinical testing
Development of new regulatory approaches that can help improve predictivity–and potentially replace, reduce and/or refine animal testing
- Leveraging advances in systems biology, stem cells, engineered tissues, and mathematical modeling
- Enhancing ability to predict risk and efficacy
- Examples include Microphysiological System and Organ-on-a-chip
Technology Modernization Action Plan (TMAP) to Accelerate Regulatory Decisions
TMAP approach to the use of technology for review of medical product applications and create a nimble workforce – with 3 components:
- Modernization of the FDA’s technical infrastructure and operations
- Enhancing FDA’s capabilities to develop technology products
- Communication and collaboration with external stakeholders to drive technological progress
CDRH Regulatory Science Tools to Help Assess New Medical Devices
Catalog of tools developed by CDRH Office of Science and Engineering Labs (OSEL) to help improve development and assessment of emerging medical technologies
- Should be evaluated for suitability within specific context of use
- Tool categories : Phantoms, Methods, Computational Models and Simulations
CDER Drug Safety Priorities
Report of CDER’s work to manage drug safety issues through modernized safety surveillance methods and innovative responses to safety concerns
- Key safety-related milestones and accomplishments of 2020
- Initiatives for promoting and protecting public health during the COVID-19 pandemic
- Ongoing programs such as Adverse Event Reporting System, Sentinel System, and Safe Use Initiative
- Ongoing activities to address the national opioid crisis, cancer-causing impurities in medicines, Drug Safety Communications tools
Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program
Use of Drug Development Tools (DDTs) -methods, materials, or measures- that have the potential to facilitate drug development
- ISTAND Pilot Program to expand DDT types by encouraging development of DDTs that are out of scope for existing DDT qualification programs but may still be beneficial for drug development
Examples:
- Tools that may help enable remote or decentralized trials
- Tools that may advance our understanding of drugs
- Tools that leverage digital health technologies
Image credit: FDA