Market Authorizations: NIGHWARE App for PTSD sleep disturbance, INMAZEB for Ebola treatment, REACTIV8 Implantable Neurostimulation System for pain, MAKENA withdrawal proposal
NIGHTWARE Digital Therapeutic
Nghtware Inc
INDICATION FOR USE: Temporary reduction of sleep disturbance related to nightmares in adults 22 years or older who suffer from nightmare disorder or have nightmares from post-traumatic stress disorder (PTSD)
The device should be used in conjunction with prescribed medications for PTSD and other recommended therapies for PTSD-associated nightmares and nightmare disorder
ADDRESSING UNMET NEED: New, low-risk treatment option that uses digital technology to provide temporary relief from sleep disturbance related to nightmares in PTSD patients
DESCRIPTION: Digital therapeutic software using Apple Watch and Apple iPhone
- Throughout the night, Apple Watch sensors monitor body movement and heart rate during sleep
- Data are sent to the Nightware server and, using a proprietary algorithm, the device creates a unique sleep profile for the patient
- When Nightware detects patient experiencing a nightmare based on analysis of heart rate and body movement, the device provides vibrations through the Apple Watch
EFFECTIVENESS AND SAFETY:
- Randomized, sham-controlled trial of 70 patients for 30 days
- Patients in the sham group wore the device, but no vibratory stimulation was provided
- Effectiveness was assessed by Sleep based on two versions of the Pittsburgh Sleep Quality Index scale, including a version intended for PTSD patients
- Safety was assessed using validated measurements of suicidality and sleepiness
- Both the sham and active groups showed improvement on the sleep scales, with the active group showing greater improvement than sham
- Nightware is intended to be used under the supervision of a healthcare provider. Patients who have been known to “act out” during their nightmares (sleepwalking, violence) should not use Nightware
REGULATORY PATHWAY: De Novo pathway with Breakthrough Designation
INMAZEB™ (atoltivimab, maftivimab, and odesivimab-ebgn) injection
Regeneron Pharmaceuticals
INDICATION: Treatment of infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection
ADDRESSING UNMET NEED: First FDA-approved treatment for Zaire ebolavirus infection in adult and pediatric patients.
MECHANISM OF ACTION: Targets the glycoprotein on the surface of Ebola virus and blocks attachment and entry of the virus.
EFFICACY:
- Clinical trial led by the U.S. National Institutes of Health and the Democratic Republic of the Congo (DRC) Institut National de Recherche Biomédicale
- Multi-center, open-label, randomized controlled trial, n= 382 adult and pediatric patients with confirmed Zaire ebolavirus infection during an Ebola virus outbreak in 2018-2019 in DRC, Inmazeb (50 mg of each monoclonal antibody) vs control
- Primary efficacy endpoint: 28-day mortality
- With Inmazeb, 33.8% died after 28 days vs. 51% on control
SAFETY:
- Most common: fever, chills, tachycardia (fast heart rate), tachypnea (fast breathing), and vomiting; however, these are also common symptoms of Ebola
- Avoid concurrent administration of a live vaccine due to the treatment’s potential to reduce the vaccine’s efficacy.
REGULATORY PATHWAY: BLA
- Orphan Drug designation, Breakthrough Therapy designation
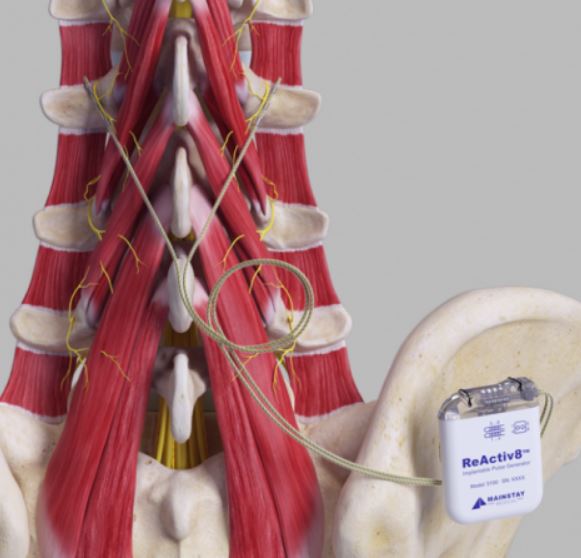
REACTIV8 Implantable Neurostimulation System
Mainstay Medical
INDICATON FOR USE: For bilateral stimulation of the L2 medial branch of the dorsal ramus as it crosses the L3 transverse process as an aid in the management of intractable chronic low back pain associated with multifidus muscle dysfunction, as evidenced by imaging or physiological testing in adults who have failed therapy including pain medications and physical therapy and are not candidates for spine surgery.
ADDRESSING UNMET NEED: Treatment option for patients who have failed therapy including pain medications and physical therapy and are not candidates for spine surgery
DEVICE DESCRIPTION: The system consists of an Implantable Pulse Generator (IPG), Leads, surgical tools and accessories, Application Software, Programmer Wand, Activator, Magnet and Tunneler. The ReActiv8 IPG, Torque Wrench and Leads, and the Mainstay Tunneler have been sterilized using ethylene oxide (EO) gas.
EFFECTIVENESS & SAFETY:
- International, multi-center, prospective, randomized, blinded trial; ReActiv8 System (Treatment group) vs. active Sham Control, n= 204
- Although the primary efficacy endpoint was not met at the 120-day visit, totality of evidence supports clinical benefit
- Additional potential secondary benefits were observed, with improvement in patient pain symptoms, as seen in the percent pain relief, and improvement in functionality measured by the Oswestry Disability Index
- System was determined to have safe electrical and mechanical characteristics
- Reasonable expectation of biocompatibility
- Adverse events that were reported were consistent with those reported with the marketed spinal systems
REGULATORY PATHWAYL PMA
- Device Generic Name: Stimulator, neuromuscular, lower back muscles, totally implanted for pain relief
- Device Procode: QLK
MAKENA (hydroxyprogesterone caproate injection) proposed withdrawal
FDA has issued a notice of opportunity for a hearing (NOOH) for withdrawal of MAKENA, marketed by Amag Pharmaceuticals
- Accelerated approval granted to Amag Pharmaceuticals in 2011 to reduce the risk of preterm birth in women who previously had a spontaneous (unexplained) preterm birth
- Company required to conduct a clinical trial to confirm the drug provided clinical benefit to newborns
- However, confirmatory study failed to substantiate efficacy
- Health Care Professionals discuss Makena’s benefits, risks and uncertainties with their patients to decide whether to use while a final decision is being made
Image credits: Nightware, Regeneron, Mainstay, Amag


