COVID-19: Fraudulent products, Reference panel comparative data for assays, First Point-of-Care (POC) antibody test, Senate testimony, Infusion pump umbrella EUA revoked

Video: Beware of Fraudulent Coronavirus Tests, Vaccines and Treatments
Video explains:
- There are currently no FDA-approved drugs or vaccines to treat or prevent COVID-19
- Products that fraudulently claim to cure, treat, diagnose, or prevent COVID-19 haven’t been evaluated by the FDA for safety and effectiveness
- They might be dangerous to you and your family
- Fraudulent products can be reported to the FDA
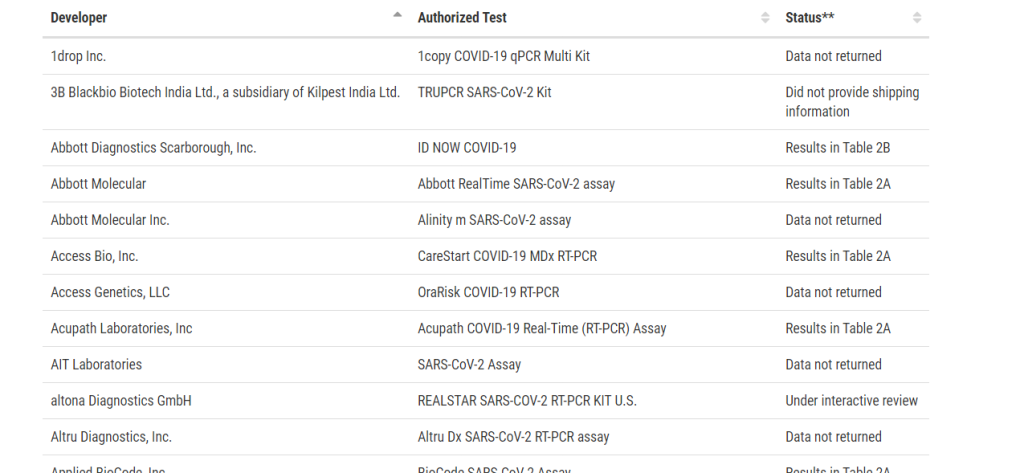
SARS-CoV-2 Reference Panel Comparative Data
From February through the middle of May, the FDA issued a total of 59 EUAs for IVDs for the qualitative detection of nucleic acid from SARS-CoV-2
- Reference Panel established to more precisely compare the performance of assays
- Composed of standardized material, suitable for the determination and direct comparison of analytical sensitivity and cross-reactivity of nucleic acid-based SARS-CoV-2 assays

First Point-of-Care (POC) Antibody Test for COVID-19: Assure COVID-19 IgG/IgM Rapid Test Device
EUA USE: For POC use using fingerstick blood samples
ADDRESSING UNMET NEED: Fingerstick blood samples can now be tested in POC settings like doctor’s offices, hospitals, urgent care centers and emergency rooms rather than having to be sent to a central lab for testing
DESCRIPTION:
- Lateral flow assay and is authorized for use with venous whole blood, serum, plasma and fingerstick whole blood
- The test is authorized for the qualitative detection and differentiation of antibodies against SARS-CoV-2 indicating recent or prior SARS-CoV-2 (COVID-19) infection
- This EUA authorizes the test for direct use with fingerstick blood samples in patient care settings, like doctors’ offices, hospitals, urgent care centers, and emergency rooms, rather than the samples being sent to a central laboratory for testing
- Serology test results should not be interpreted to mean that a patient is immune to the virus or as an indication to stop taking steps to protect themselves and others against the spread of COVID-19

Testimony: Senate Committee on Health, Education, Labor and Pensions
Hearing entitled, “COVID-19: An Update on the Federal Response.” FDA Commissioner testimony described Agency’s efforts to protect the American public, help ensure the safety, efficacy, and quality of FDA-regulated medical products, and provide industries with tools and flexibility to do the same
- Diagnostic testing
- Vaccine development
- Therapeutic development
- Medical product supply (drugs & biological products, medical devices,
- Inspections
- Food supply
- Fraudulent products

Umbrella Emergency Use Authorization of Infusion Pumps Infusion Pump Accessories Revoked
FDA issued an umbrella EUA for infusion pumps and infusion pump accessories in May
- For use by healthcare providers to treat COVID-19 conditions caused
- For controlled infusion of medications, total parenteral nutrition (TPN), and/or other fluids
- Included infusion pumps with remote monitoring or remote manual control features or administration sets and other accessories
- However, no device has been added to the list of authorized devices
Current circumstances support revocation of the umbrella EUA
- Individual EUAs will allow for tailored indications and scopes of authorization
Image credit: FDA