Other News and Views: Detection of nitrosamine contaminants, Trial stimulation before spinal cord stimulator implantation, Tanning beds and booths, Right to Try act, Naloxone prescribing
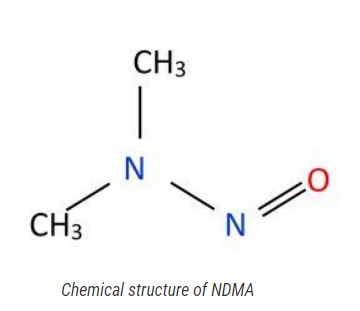
Rigorous Detection of Nitrosamine Contaminants in Metformin Products: Balancing Product Safety and Product Accessibility
Since 2018, multiple drug products, including angiotensin receptor blockers (ARBs), histamine blocker ranitidine (commonly known as Zantac), have been recalled due to the presence of nitrosamines at unacceptable amounts
- CDER scientists have developed and publicly shared gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-MS (LC-MS) technologies
- For detecting and quantifying up to eight different nitrosamines at levels below acceptable U.S. intake limit for the nitrosaminat low levels
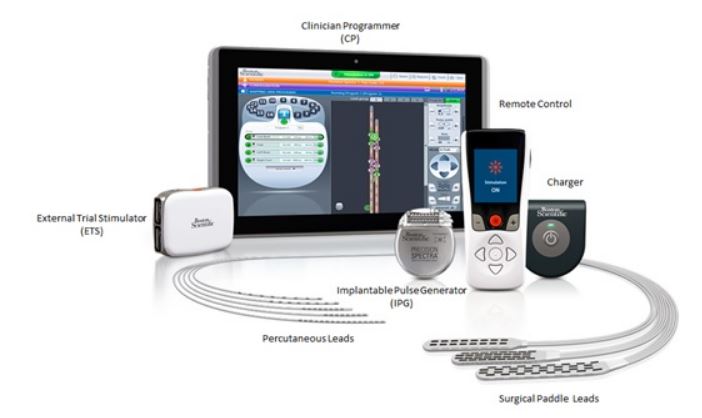
Conduct a Trial Stimulation Period Before Implanting a Spinal Cord Stimulator (SCS)
Implanted SCS are an aid to cope with unmanageable, chronic chronic pain
- However, FDA continues to receive reports of associated serious side effects
Health care providers should conduct a trial stimulation period with patients to confirm satisfactory pain relief before implanting a spinal cord stimulator (SCS)
- Implant only in patients who have passed stimulation trial performed for 3-7 days showing 50% percent reduction in pain symptoms.
- Discuss the benefits and risks of the different types of implants and other treatment options with patients
- Several other recommendations

Tanning Beds and Booths
Tanning beds and booths are sunlamp products used for indoor UV skin tanning
- Risk of fire due to improper maintenance, dirty air filters blocking air flow, incompatible parts, failure to perform servicing and maintenance
- Owners and operators should perform maintenance recommended by product manufacturers to reduce risk of smoke and fire
- FDA monitoring adverse event reports and will keep public informed

New Rule on Reporting Requirements for Right to Try Act
Right to Try Act provides pathway for patients with life-threatening diseases who have tried all approved treatment options and who are unable to participate in a clinical trial, to access certain unapproved treatments
- Sponsor or manufacturer of drug/biologic is responsible for determining whether to make their product available to patients who qualify
- New statutory requirement for sponsors and manufacturers to provide an annual summary to the FDA

Labeling Changes Regarding Naloxone
Naloxone can be administered by individuals with or without medical training to help reduce opioid overdose deaths
- Required labeling changes recommend health care professionals prescribe naloxone when they prescribe medicines to treat OUD
- Also, they consider prescribing naloxone to patients being prescribed opioid pain medicines who are at increased risk of opioid overdose
Image credit: FDA