Market Authorizations: 2019-nCoV diagnostic, ERVEBO Ebola vaccine, TEPEZZA for thyroid eye disease
 2019-nCoV Real-Time RT-PCR Diagnostic Panel
2019-nCoV Real-Time RT-PCR Diagnostic Panel
CDC
INTENDED USE: For the presumptive qualitative detection of nucleic acid from the 2019-nCoV in upper and lower respiratory specimens (such as nasopharyngeal or oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) collected from individuals who meet CDC criteria for 2019-nCoV testing. Testing is limited to qualified laboratories
designated by CDC and, in the United States, certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), to perform high complexity tests.
PREPARATION & TESTING:
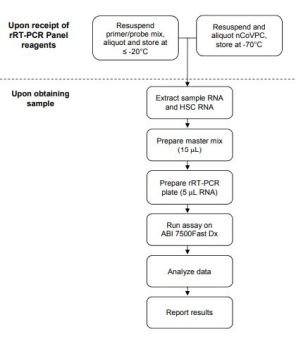
DESCRIPTION:
- Reverse transcriptase polymerase chain reaction (PCR) test that provides presumptive detection of 2019-nCoV from respiratory secretions, such as nasal or oral swabs
- Positive test result indicates likely infection with 2019-nCoV and infected patients should work with their health care provider to manage their symptoms and determine how to best protect the people around them
- Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions; negative results must be combined with clinical observations, patient history and epidemiological information
REGULATORY PATHWAY: Emergency Use Authorization to address public health emergency

ERVEBO ebola vaccine for intramuscular injection
Merck
INDICATION: Prevention of disease caused by Zaire ebolavirus in individuals 18
years of age and older
ADDRESSING UNMET NEED: First FDA-approved vaccine for the prevention of Ebola virus disease (EVD), caused by Zaire ebolavirus
MECHANISM OF ACTION: Immunization results in an immune response and protection from disease caused by Zaire ebolavirus
EFFECTIVENESS:
- Guinea study, during 2014-2016 outbreak, n= 3,537 contacts and contacts of contacts with laboratory-confirmed EVD, “immediate” or 21-day “delayed” vaccination
- ERVEBO 100% effective in preventing Ebola cases with symptom onset greater than 10 days after vaccination
- No EVD cases in “immediate” cluster group vs 10 cases in 21-day “delayed” cluster group
- Liberia study- n= 477, Sierra Leone study – n=500, Canada, Spain, US study – n=900
- Antibody responses in Canada, Spain and U.S. study were similar to those among individuals in the studies conducted in Liberia and Sierra Leone.
SAFETY:
- Most commonly reported side effects were pain, swelling and redness at the injection site, as well as headache, fever, joint and muscle aches and fatigue.
REGULATORY PATHWAY: BLA
- Priority Review and Tropical Disease Priority Review Voucher, Breakthrough Therapy designation
- Because of public health importance, FDA worked closely with Merck and completed its BLA evaluation in less than six months

TEPEZZA (teprotumumab-trbw) injection, for intravenous use
Horizon Therapeutics
INDICATION: Treatment of Thyroid Eye Disease
ADDRESSING UNMET NEED:
- First drug approved for the treatment of thyroid eye disease-
- Rare condition where muscles and fatty tissues behind eye become inflamed, causing eyes to be pushed forward and bulge outwards (proptosis)
MECHANISM OF ACTION: Not been fully characterized- binds to IGF-1R and blocks activation and signaling
EFFICACY:
- Two studies, n=170 patients with active thyroid eye disease, TEPEZZA or placebo
- Primary endpoint: >2 millimeter reduction in proptosis- 71% (Study 1) and 83% (Study 2) vs. 20% and 10%
SAFETY:
- Most common adverse reactions: Muscle spasm, nausea, alopecia, diarrhea, fatigue, hyperglycemia, hearing loss, dry skin, dysgeusia, headache
- Should not be used if pregnant, counseling on pregnancy prevention during treatment and for 6 months following last dose
REGULATORY PATHWAY: BLA
- Priority Review, in addition to Fast Track and Breakthrough Therapy Designation
- Orphan Drug designation, with support from FDA Orphan Products Grants Program
- Postmarketing commitments: Additional studies to characterize efficacy, safety, quality
Image credits: FDA, Merck, Horizon Therapeutics