
Advance Development of Novel Coronavirus Medical Countermeasures (MCM)
- Expedite development and availability of MCM to diagnose, treat, mitigate, prevent
- Utilize pathways, including Emergency Use Authorization (EUA) to more rapidly make MCM available
- Provide regulatory advice, guidance, and technical assistance to sponsors developing investigational MCMs
- Provide review and feedback on development proposals including design and set-up of clinical trials
- Protect safety of nation’s blood supply and human cells, tissues,cellular/tissue-based products for transplantation
- Enable access to investigational MCM through EUA or expanded access mechanisms
- Protect consumers against fraudulent products by monitoring fraudulent products and false product claims and taking appropriate action

2019 CDER New Drug Approvals Report
Annual report entitled Advancing Health Through Innovation: New Drug Therapy Approvals
- Variety of novel drugs for advancing patient care – never approved in US
- Overview of other notable approvals — new uses of uses for approved drugs
- Treat new population of patients, such as children
- Innovative ways to enhance efficiency and expedite review and approval
Innovation and Access areas
- Rare Diseases
- Neurological and Psychiatric disorder
- Infectious diseases
- Heart, Lung, Circulatory, Endocrine diseases
- Autoimmune conditions
- Women’s and Men’s specific health issues
- Cancers and blood disorders
- Biosimilars
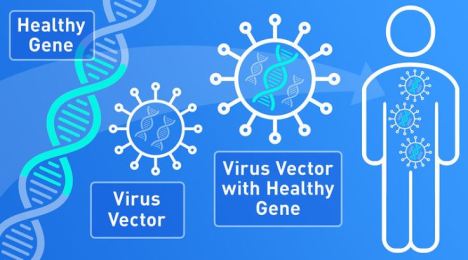
Strong Support of Innovation in Development of Gene Therapy Products
FDA efforts to support innovators developing new gene therapy products, which insert new genetic material into a patient’s cells
- 4 products approved
- > 900 investigational new drug (IND) applications for ongoing clinical studies
- will serve to improve therapeutic choices
Publication of guidances issued to provide recommendations for product developers
- Draft Guidance: Interpreting Sameness of Gene Therapy Products Under the Orphan Drug Regulations
- Final Guidance: Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs)
- Final Guidance: Testing of Retroviral Vector-Based Gene Therapy Products for Replication Competent Retrovirus (RCR) during Product Manufacture and Patient Follow-up
- Final Guidance: Long Term Follow-Up After Administration of Human Gene Therapy Products
- Final Guidance: Human Gene Therapy for Hemophilia
- Final Guidance: Human Gene Therapy for Retinal Disorders
- Final Guidance: Human Gene Therapy for Rare Diseases

New Efforts to Further Deter Anti-Competitive Business Practices, Support Competitive Market for Biological Products to Help Americans
FDA and FTC to collaborate to curtail and discourage anti-competitive behavior to faciliate robust competition and bring down cost for biologics
- Promote greater competition in biologic markets
- Deter behavior that impedes access to samples needed for the development of biologics, including biosimilars
- Take appropriate action against false or misleading communications
- Review patent settlement agreements involving biologics, including biosimilars, for antitrust violations
 Heart Health Education for Women
Heart Health Education for Women
- Heart health educational video ‘Getting a Beat : On what women know about heart health
- New KNOWH the Difference initiative, which focuses on sharing important knowledge and news on women’s health (KNOWH)
- OWH research and extramural funding opportunities
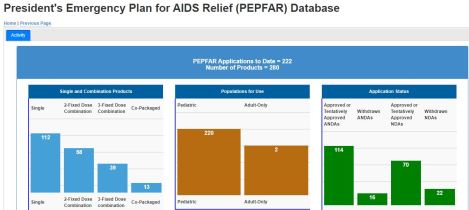
Mobile-Friendly Database with Information on Life-Saving HIV Drugs
Interactive database on critical information about antiretrovirals (ARVs) eligible for purchase under the President’s Emergency Plan for AIDS Relief (PEPFAR) program
- Empower public and health care providers by enhancing the amount and availability of information and data provided on each drug
- Health care providers, consumers, procurer access to FDA-reviewed product labeling and essential scientific information for safe and effective use of drug
- Information on pediatric drugs, where manufactured, shelf-life, storage requirements
- Ability to export reports, collect metrics, readily access on mobile platforms

FDA Warns Maker of Nicotine-Containing Toothpicks of Several Violations, Including Illegal Sales
- Warning letter to Smart Toothpicks LLC, Tempe, Arizona, for selling dissolvable tobacco products, including Peppermint Ice Nicotine Toothpicks
- Three specific violations:
- selling a tobacco product to a minor through the company’s website
- selling unauthorized modified risk tobacco products
- failing to include required nicotine warning statements on both packaging and advertising
- Written response to this letter within 15 working days on corrective actions
- discontinue violative labeling, advertising, sale, and/or distribution
- plan for maintaining compliance with the FD&C Act
Action is part of the agency’s Youth Tobacco Prevention Plan, to prevent and reduce youth tobacco use
Image credits: FDA