Market Authorizations: IB-STIM for Irritable Bowel Syndrome, PIQRAY for advanced or metastatic breast cancer, ZOLGENSMA for spinal muscular atrophy
 IB-STIM
IB-STIM
Innovative Health Solutions
INDICATION FOR USE: Percutaneous electrical nerve field stimulator (PENFS) system intended to be used in patients 11-18 years of age with functional abdominal pain associated with irritable bowel syndrome (IBS). The IB-Stim is intended to be used for 120 hours per week up to 3 consecutive weeks, through application to branches of Cranial Nerves V, VII, IX and X, and the occipital nerves identified by transillumination, as an aid in the reduction of pain when combined with other therapies for IBS.
ADDRESSING UNMET NEED:
- First medical device for reduction of functional abdominal pain in patients 11-18 years of age with irritable bowel syndrome (IBS) when combined with other therapies for IBS
- Advancing the development of pediatric medical devices
DESCRIPTION:
- Prescription-only device
- Small single-use electrical nerve stimulator placed behind patient’s ear
- Battery-powered chip that emits low-frequency electrical pulses to stimulate branches of certain cranial nerves continuously for five days
- Stimulating nerve bundles in and around the ear is thought to provide pain relief
- Patients can use the device for up to three consecutive weeks to reduce functional abdominal pain associated with IBS
EFFECTIVENESS & SAFETY:
- Clinical study, n= 50 patients 11-18 years of age with IBS; IB-Stim vs placebo
- Endpoint: Change from baseline to the end of the third week in worst abdominal pain, usual pain and Pain Frequency Severity Duration (PFSD) scores
- Worst pain at baseline: Similar between the treatment and placebo groups
- Greater change (improvement) in worst pain from baseline to week three in IB-Stim group; effect also seen at weeks one and two
- Greater change also demonstrated in composite PFSD scores from baseline to week three in the IB-Stim group compared to the placebo group.
- 30% decrease in usual pain at the end of three weeks in 52% IB-Stim patients vs 30% placebo
- Six patients reported mild ear discomfort and three patients reported adhesive allergy at the site of application
- Contraindicated for patients with hemophilia, patients with cardiac pacemakers or those diagnosed with psoriasis vulgaris
REGULATORY PATHWAY: De Novo request
 PIQRAY (alpelisib) tablets
PIQRAY (alpelisib) tablets
Novartis
INDICATION: In combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CAmutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
- Also approved the companion diagnostic test: Therascreen PIK3CA RGQ PCR Kit to detect the PIK3CA mutation in a tissue and/or a liquid biopsy
- Patients who are negative by the therascreen test using the liquid biopsy should undergo tumor biopsy for PIK3CA mutation testing.
ADDRESSING UNMET NEED: First PI3K inhibitor to demonstrate a clinically meaningful benefit in treating patients with this type of breast cancer.
MECHANISM OF ACTION: Inhibitor of phosphatidylinositol-3-kinase (PI3K) with activity predominantly against PI3Kα; inhibited the phosphorylation of PI3K downstream targets, induce an increase in estrogen receptor (ER) transcription in breast cancer cells
EFFICACY:
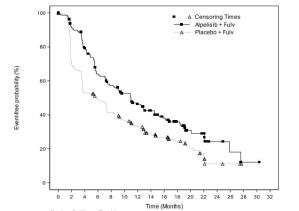
- Randomized trial, n=572 postmenopausal women and men with HR-positive, HER2-negative, advanced or metastatic breast cancer
- Addition of Piqray to fulvestrant significantly prolonged progression- free survival (median of 11 months vs. 5.7 months) in patients whose tumors had a PIK3CA mutation.
SAFETY:
- Common side effects: High blood sugar levels, increase in creatinine, diarrhea, rash, decrease in lymphocyte count in the blood, elevated liver enzymes, nausea, fatigue, low red blood cell count, increase in lipase (enzymes released by the pancreas), decreased appetite, stomatitis, vomiting, weight loss, low calcium levels, aPTT prolonged (blood clotting taking longer to occur than it should), hair loss
- Monitor patients for severe hypersensitivity reactions (intolerance)
REGULATORY PATHWAY: NDA
- First novel drug approved under the Real-Time Oncology Review pilot program
- Use of updated Assessment Aid, a multidisciplinary FDA review template for review consistency and reduced review time
- Priority Review designation
- Postmarketing Commitments: Overall survival data, drug interaction studies
 ZOLGENSMA (onasemnogene abeparvovec-xioi) intravenous infusion
ZOLGENSMA (onasemnogene abeparvovec-xioi) intravenous infusion
AveXis
INDICATION: Adeno-associated virus vector-based gene therapy indicated for the treatment of pediatric patients less than 2 years of age with spinal muscular atrophy *SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene
Limitations for use:
- Safety and effectiveness of repeat administration not been evaluated
- Use in patients with advanced SMA not been evaluated
ADDRESSING UNMET NEED: First gene therapy approved to treat children less than two years of age with spinal muscular atrophy (SMA), a leading genetic cause of infant mortality
MECHANISM OF ACTION: Recombinant AAV9-based gene therapy designed to deliver copy of gene encoding the human SMN protein. SMA is caused by a bi-allelic mutation in the SMN1 gene, which results in insufficient SMN protein expression. Intravenous administration of ZOLGENSMA results in cell transduction and expression of SMN protein has beennobserved in two human case studies
EFFICACY:
- Ongoing clinical trial and a completed clinical trial, n=36, patients with infantile-onset SMA, 2 weeks -8 months old
- 19/ 21 patients remaining, age:9.4 -18.5 months; 13/19 at least 14 months of age
- Compared to natural history of patients with infantile-onset SMA, patients treated with Zolgensma demonstrated significant improvement in ability to reach developmental motor milestones (e.g., head control and the ability to sit without support)
SAFETY:
- Boxed Warning: Acute liver injury
- Most common side effects: Elevated liver enzymes and vomiting
REGULATORY PATHWAY: BLA
- Fast Track, Breakthrough Therapy, and Priority Review designations
- Orphan Drug designation and rare pediatric disease priority review voucher,
Image credit: Innovative Health Solutions, Novartis, AveXis