 Vaccination Is the Best Protection Against Measles
Vaccination Is the Best Protection Against Measles
Measles, Mumps, and Rubella Virus Vaccine Live (M-M-R II, commonly called MMR)
- For people ages 12 months and older
- Two doses of the vaccine is about 97% effective at preventing measles
Measles, Mumps, Rubella and Varicella Virus Vaccine Live (ProQuad, commonly called MMRV)
- For children ages 1 through 12 years.
- Also prevents chickenpox
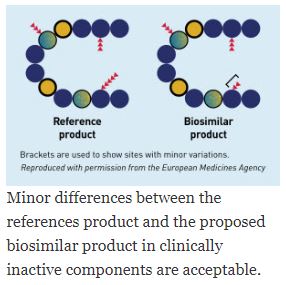 Policy advancements to help bring interchangeable biosimilars to market
Policy advancements to help bring interchangeable biosimilars to market
Pathway for “interchangeable” biologics
- Substituted without the involvement of the prescriber
- Similar to how generic drugs are routinely substituted for brand name drugs
Final guidance on the pathway for “interchangeable” biologics
- Overview of important scientific considerations in demonstrating interchangeability
- Biological product is biosimilar to reference product and produces same clinical result
- Risk of safety or diminished efficacy of alternating or switching will not be greater than the risk of using the reference product without such alternation or switch
 Actions for violations by online posts on flavored e-liquids by social media influencers
Actions for violations by online posts on flavored e-liquids by social media influencers
FDA and FTC warning letters to four firms that manufacture, sell, advertise flavored e-liquid products for social media influencers
- Violations related to online posts by social media influencers on each company’s behalf, including failure to include the required nicotine warning statement
- Enforcement action to address increased popularity of e-cigarettes among youth leading to health concerns, nicotine addiction, and more likely to transition to conventional cigarettes
 Project Facilitate to assist physicians seeking access to unapproved therapies for patients with cancer
Project Facilitate to assist physicians seeking access to unapproved therapies for patients with cancer
New pilot program to assist oncology health care professionals in requesting access to unapproved therapies for patients with cancer
- New call center called Project Facilitate as a single point of contact
- Help submit Expanded Access request for an individual patient, including follow-up of patient outcomes
- Expanded Access is pathway for treatment by investigational treatment outside of clinical trials for immediately life-threatening or serious disease or condition
- Expanded Access requests help weigh whether potential benefit of investigational treatment justifies the potential risks

Draft Guidance for Industry on Enhancing the Diversity of Clinical Trial Populations
- More inclusive trial practices, trial designs, and methodological approaches to broaden eligibility criteria
- Increase enrollment of more diverse populations in clinical trials
- Maximizes generalizability of trial results
- Better understand therapy’s benefit-risk profile across the patient population likely to use the drug in clinical practice, without jeopardizing patient safety
- Reflects FDA policy encouraging inclusion in clinical trials of participants representative of the broad population of patients including minorities
 Fixed-quantity blister packaging for certain opioid pain medicines to help decrease unnecessary exposure to opioids
Fixed-quantity blister packaging for certain opioid pain medicines to help decrease unnecessary exposure to opioids
Misuse of prescription opioids by getting them from a friend or family member
- >191 million opioid prescriptions dispensed to ~ 60 million patients, either as first-time prescriptions or refills; ~ 90% patients reported not finishing what was prescribed to them
Special opioid packaging for reducing unused doses available for misuse, abuse, inappropriate access, accidental poisoning or overdose- considertions
-
Actual opioid use compared to prescribed amounts for common surgical procedures and acute pain conditions
-
5-, 10-, and 15-Count Blister Packages of Certain IR Opioid and IR Opioid/Acetaminophen Products
-
New Packaging/Disposal REMS Element
-
Safety-Enhancing Benefits of Fixed-Quantity Blister Packaging for Opioid Analgesics for Treatment of Acute Pain
- National Academies of Sciences, Engineering, and Medicine (NASEM) to help advance guideline development
“Remove the Risk” campaign
 Tracking and Acting on Safety Data Throughout a Drug’s Lifecycle
Tracking and Acting on Safety Data Throughout a Drug’s Lifecycle
Agency tracks, analyzes and acts on the adverse event data collected over time to discover and communicate new risks associated with approved drugs
- Reports from patients, health care professionals and manufacturers about adverse events in FDA Adverse Event Reporting System (FAERS)
- Monitor FAERS database to identify safety signals, analyze and interpret data, combine with other scientific information for decision-making
- If new risk confirmed, require addition to product’s label and may require a Risk Evaluation Mitigation Strategy (REMS) to ensure benefits outweigh risks
- From 2002 through 2014, new safety issues discovered and added to labels for 278 drugs
 Participation in 2019 Test Plan for Digital Health Software Precertification (Pre-Cert) Program
Participation in 2019 Test Plan for Digital Health Software Precertification (Pre-Cert) Program
Agency seeking test cases from software organizations planning to submit a De Novo Request or 510(k) submission for software as a medical device (SaMD) to meet the goals of the Test Plan
- Small and large software development firms
- Companies that develop a range of products, including both low- and high-risk SaMD
- Companies that are not considered to be traditional medical device manufacturers but who intend to make SaMD
 Helping to Better Communicate the Risks and Benefits in Prescription Drug Advertising
Helping to Better Communicate the Risks and Benefits in Prescription Drug Advertising
Direct-to-consumer advertising influence awareness of prescription drug treatments, influence patients and doctors
- Using eye-tracking technology, researchers are examining elements of these ads that can affect the viewer’s ability to understand risks and benefits
- With high-distraction ad – less able to recall or recognize risks described, spent less time on written statement of risks
- Objective to help drug companies develop ads that better inform public
 Assuring Drug Quality Around the Globe
Assuring Drug Quality Around the Globe
Programs to address the challenges to drug quality posed by globalization to protect patients and consumers
- Tools to assure continued safety, effectiveness, quality post-approval
- Same quality for both domestic and foreign manufactured products
- Addressing quality inspections and issues
Image credit: F DA