
EVENITY (romosozumab-aqqg) injection, for subcutaneous use
Amgen
INDICATION FOR USE: Treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy
Limitations of Use : Anabolic effect wanes after 12 monthly doses of therapy. Therefore, the duration of EVENITY use should be limited to 12 monthly doses. If osteoporosis therapy remains warranted, continued therapy with an anti-resorptive agent should be considered
- > 10 million US women osteoporosis; weakened bones likely to fracture
- Approval provides women with postmenopausal osteoporosis with high risk of fracture with a new treatment
MECHANISM OF ACTION: Monoclonal antibody that inhibits action of sclerostin, a regulatory factor in bone metabolism; increases bone formation and, to a lesser extent, decreases bone resorption
- 2 randomized, double-blind studies, placebeo and alendronate controlled respectively, n>11,000 women with postmenopausal osteoporosis
- Endpoints: Risk of a new fracture in spine (vertebral fracture): 73% lowered risk vs. placebo, maintained over the second year of trial when Evenity. 50% lowered risk vs. two years of alendronate alone
- Evenity followed by alendronate reduced risk of fractures in other bones (nonvertebral fractures) vs. alendronate alone
- Boxed Warning: Potential risk for myocardial infarction, stroke and cardiovascular death
- Common side effects: Joint pain, headache, injection site reactions
- Pediatric assessments: Waived; do not apply to population
- Postmarketing requirements: Cardiovascular safety

DENGVAXIA (Dengue Tetravalent Vaccine, Live) Suspension for Subcutaneous Injection
Sanofi Pasteur
INDICATION FOR USE: indicated for the prevention of dengue disease caused by dengue virus serotypes 1, 2, 3 and 4. DENGVAXIA is approved for use in individuals 9 through 16 years of age with laboratory-confirmed previous dengue infection and living in endemic areas
Limitations of use: Not approved for use in individuals not previously infected by any dengue virus serotype or for whom this information is unknown; Safety and effectiveness have not been established in individuals living in dengue non-endemic areas who travel to dengue endemic areas
ADDRESSING UNMET NEED:
- FDA committed to working with CDC and WHO to combat public health threats
- Approval is important step toward reducing impact of virus in endemic US regions
MECHANISM OF ACTION: Elicits dengue-specific immune responses against four dengue virus serotypes
EFFICACY:
- Three randomized, placebo-controlled studies, n=35,000 individuals in dengue-endemic areas, including Puerto Rico, Latin America and Asia Pacific region, 9-16 years of age
- Endpoint: Symptomatic virologically-confirmed dengue (VCD); DENGVAXIA 76% effective in preventing VCD in individuals who previously had laboratory- confirmed dengue disease
SAFETY:
- Most commonly reported side effects: Headache, muscle pain, joint pain, fatigue, injection site pain and low-grade fever
REGULATORY PATHWAY: BLA
- Priority Review and a Tropical Disease Priority Review Voucher
- Postmarketing Study Requirement/Commitments: Safety and effectiveness in children 2 to < 9 years of age

MAVYRET (glecaprevir and pibrentasvir) tablets
AbbVie
INDICATION FOR USE: Treatment of adult and pediatric patients 12 years and older or weighing at least 45 kg with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A)
ADDRESSING UNMET NEED: First treatment for all genotypes of hepatitis C in pediatric patients
MECHANISM OF ACTION: Fixed-dose combination of glecaprevir and pibrentasvir, which are direct-acting antiviral agents against the hepatitis C virus
EFFICACY:
- Open-label study, n=47 adolescent subjects 12 –< 18 years with genotype 1, 2, 3 or 4 HCV infection without cirrhosis or with mild cirrhosis, 8 or 16 weeks.
- 100% had no virus detected in blood 12 weeks after finishing treatment, suggesting infection had been cured
SAFETY:
- Adverse reactions observed were consistent with those observed in adults
- Most commonly reported adverse reactions: Headache and fatigue
REGULATORY PATHWAY: Supplemental NDA
- Initial US approval (NDA) in adult patients in 2017

BENLYSTA (belimumab) intravenous infusion
GlaxoSmithKline
INDICATION FOR USE: Treatment of patients aged 5 years and older with active, autoantibody-positive, systemic lupus erythematosus (SLE) who are receiving standard therapy
ADDRESSING UNMET NEED:
- First treatment for pediatric patients with Systemic Lupus Erythematosus (SLE)
- Benlysta has been approved for use in adult patients since 2011
EFFICACY:
- International, randomized, doubleblind, placebo-controlled, 52-week, n=93 pediatric patients with a clinical diagnosis of SLE
- Primary efficacy endpoint: SLE Responder Index (SRI-4) at Week 52; numerically higher patients achieving a response in SRI-4 with BENLYSTA vs. placebo
SAFETY:
- Warning for mortality, serious infections, hypersensitivity and depression
- Most common side effects: Nausea, diarrhea and fever, infusion reactions
REGULATORY PATHWAY: sBLA, Priority review

TIBSOVO (ivosidenib) tablets
Agios Pharmaceuticals
RealTime IDH1 Assay
Abbott
INDICATION FOR USE: Treatment of newly-diagnosed acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test in adult patients who are ≥ 75 years old or who have comorbidities that preclude use of intensive induction chemotherapy
ADDRESSING UNMET NEED: First-line treatment for AML with IDH1 mutation
EFFICACY:
- Open-label, single-arm, multicenter clinical trial, n=28 (newly-diagnosed AML with IDH1 mutation detected by Abbott RealTimeTM IDH1 Assay
- Endpoints: Rate of complete remission (CR) or complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, conversion rate from transfusion dependence to transfusion independence
- CR+CRh :42.9%, 41.2% achieved transfusion independence lasting at least 8 weeks
SAFETY:
- Boxed Warning for Differentiation Syndrome which may be life-threatening or fatal
- Adverse reactions: Diarrhea, fatigue, edema, decreased appetite, leukocytosis, nausea, arthralgia, abdominal pain, dyspnea, differentiation syndrome and myalgia
REGULATORY PATHWAY: sNDA
- Used Real-Time Oncology Review pilot program
- Priority Review and Orphan Product designation
- Postmarketing requirement: Determine safe dose in mild, moderate and severe hepatic impairment

VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) capsules
FoldRx (Pfizer subsidiary)
INDICATION FOR USE: Treatment of cardiomyopathy of wild type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization
ADDRESSING UNMET NEED:
- Transthyretin-mediated amyloidosis is rare, debilitating, often fatal disease
- Approval an important advancement in treatment of cardiomyopathy caused by transthyretin-mediated amyloidosis
MECHANISM OF ACTION: Selective stabilizer of TTR; slows dissociation into monomers, the rate-limiting step in the amyloidogenic process
EFFICACY:
- Multicenter, international, randomized, double-blind, placebo-controlled study
n=441 patients with wild type or hereditary ATTR-CM - Endpoints: All-cause mortality and frequency of cardiovascular-related hospitalizations
- Significant reduction (p=0.0006) in all-cause mortality and frequency of
cardiovascular-related hospitalizations with Vyndaqel / Vyndamax group
SAFETY:
- No drug-associated side effects identified
- May cause fetal harm when administered to pregnant woman
REGULATORY PATHWAY: NDA
- Fast Track, Priority Review and Breakthrough Therapy designations
- Orphan Drug designation
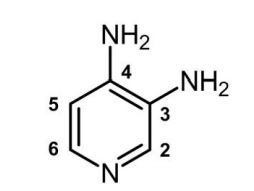
RUZURGI (amifampridine) tablets
Jacobus Pharmaceutical Company
INDICATION FOR USE: Treatment of Lambert-Eaton myasthenic syndrome (LEMS) in patients 6 to less than 17 years of age
ADDRESSING UNMET NEED:
- LEMS is a rare autoimmune disorder affecting connection between nerves and muscles
- First approved treatment for LEMS specifically in children
MECHANISM OF ACTION: Broad spectrum potassium channel blocker
EFFICACY:
- Randomized, double-blind, placebo-controlled withdrawal study
- Primary measure of efficacy: Change in Triple Timed Up and Go test (3TUG) and self-assessment scale for LEMS-related weakness
- Less impairment with Ruzurgi; greater perceived weakening with placebo
SAFETY:
- Most common side effects: Burning or prickling sensation (paresthesia), abdominal pain, indigestion, dizziness and nausea
- Seizures in patients without a history of seizures
REGULATORY PATHWAY: NDA, Priority Review and Fast Track designations, Orphan Drug designation
Image credits: Amgen, Sanofi, AbbVie, Agios, GSK, Pfizer, Jacobus