Team-Based Approach to Medical Device and Radiological Product Evaluation and Quality
Operationalizing Office of Product Evaluation and Quality (OPEQ)
- Identifying, Resolving, and Communicating Safety Issues — Faster and Better
- Informing New Product Approvals with Latest Safety Data
- Advancing Safer and More Effective Innovative Technologies
- Enhancing Evidence Generation Throughout the Total Product Life Cycle
- Fostering Employee Engagement, Opportunity, and Success
Capturing Patient Experience Through Deep Learning
- FDA developing computational approaches to use deep learning, a form of artificial intelligence (AI), to extract adverse events of drugs and therapeutic biologics (using standard MedDRA terms) automatically from NDAs
- Patient narratives in NDAs used to train a neural network to make correct MedDRA coding decisions using diverse natural language processing (NLP)
- Development of user-friendly tool to check MedDRA coding accuracy of previous submissions, such as adverse event reports from drug developers

Using Patient Preference Information (PPI) in Medical Device Evaluation
PPI defined as
- Qualitative/Quantitative assessments of relative patient desirability or acceptability of specified alternatives or choices among outcomes or other attributes
- Clarify what benefits and risks are most important to patients
- Impact premarket clinical study design, benefit-risk assessments, postmarket evaluation
Agency has identified Priority List of Patient Preference-Sensitive Areas
Parameters for patient preference-sensitive areas
- Better understand full impact of disease and treatment options on patients
- Patients may value benefits-risks differently from healthcare professionals
- Population-level differences in patient perspectives not well understood
- Significant public health impact

CMS comprehensive strategy to foster innovation for transformative medical devices
Goal to get new innovations to beneficiaries concurrent with FDA approval
- Removing government barriers to innovation
- Harmonizing CMS coverage, coding, and payment processes
Initiatives
- CMS-FDA Parallel Review Program – FDA approval and CMS coverage on same day
- Modernize payment policies for ‘Breakthrough Devices’ designation – waive requirement for “substantial clinical improvement”
- More frequent, semi-annual, opportunities to request HCPCS code
- Consider commercial payments in determining payments for innovative Durable Medical Equipment (DME)
- Reevaluiate guidelines for new hospital technology add-on payment (NTAP)
- Enhance ease of billing for new technologies
- New guidance on Local Coverage Decisions (LCD) – contractors not authorized to make coverage determinations to automatically non-cover any item or service

Efforts to Advance the Development of Biologics
Biologics, regulated by CBER, are either living cells or tissues, or are made from them, such as stem cells and genetically engineered immune cells
- Drugs: Includes blood components/derivatives, vaccines, allergenics, and cellular/gene therapies
- Devices: In vitro diagnostic tests for screening blood supply, for manufacturing blood and tissue products
- Safety and efficacy linked to quality and consistency of manufacturing
Cellular and Gene Therapy Approvals in 2018
- LUXTURNA for heritable disorder that causes blindness: retinal dystrophy associated with mutations in the RPE65 gene
- YESCARTA, CAR-T cell therapy to treat adults with certain types of relapsed or refractory diffuse large B cell non-Hodgkin lymphoma
Vaccine and Blood Product Approvals in 2018
- HEPLISAV-B, Hepatitis B vaccine to prevent infection caused by all known subtypes of hepatitis B virus in adults 18 of age and older
- SHINGRIX, vaccine for shingles prevention in adults 50 years of age and older
- COBAS Zika Test and the PROCLIEX Zika Virus Assay for Zika detection in human plasma

Addressing Health Disparities Through Communication, Research, and Collaboration
Office of Minority Health and Health Equity (OMHHE) promotes and protects health of diverse populations and strives for health equity
- Issued guidance on the collection of race and ethnicity data in clinical trials.
- Created Minorities in Clinical Trials patient videos
- Work on sickle cell disease, a genetic condition common among African Americans
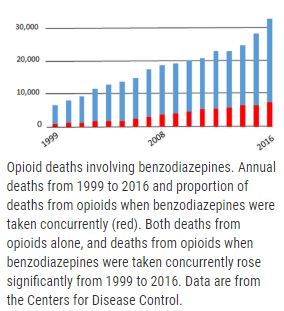
Preclinical Research to Achieve Safer Prescribing of Psychoactive Therapeutics for Patients Who Use Opioids
More than 30% of deaths from opioids involve use of benzodiazepines, commonly used for anxiety or insomnia
- Might worsen the respiratory effects of opioids in patients being treated for pain. To achieve this goal
- Gaps in evidence needed for safer prescribing
FDA developing Preclinical Model to address gaps
- Rat study and predict interaction of psychoactive sedative drugs and opioids
- Exposed to psychoactive drug with or without the opioid oxycodone
- Determine respiratory effect and opioid-induced respiratory depression
- Measurement of achieved blood concentrations over time to determine how to administer the drugs so that tested drug and opioid reach simultaneous peak levels

Marketed Device Safety Measures
SURGICAL MESH for transvaginal repair of pelvic organ prolapse – Sales stopped
- FDA ordered all manufacturers of surgical mesh intended for transvaginal repair of anterior compartment prolapse (cystocele) to stop selling-distributing immediately
- Manufacturers- Boston Scientific and Coloplast- have not demonstrated reasonable assurance of safety and effectiveness for these high risk devices these devices
SURGICAL STAPLERS- New steps to reduce risks
- Large number of adverse events reports including opening of staple line, malformation of staples, misfiring, difficulty in firing, failure of stapler to fire staple, misapplied staples
- Increasing regulatory requirements from Class I (low risk) to Class II (moderate risk)
- Require labeling to provide adequate information for use, including hazards, contraindications, information for safe and effective use
BREAST IMPLANTS- New efforts to protect health and ensure safety
- Weighing risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL)
- Improve labeling to communicated risk of BIA-ALCL, greater risk of BIA-ALCL with textured implants, and risk of developing systemic symptoms to women and health care professionals professionals
Stronger warnings about rare but serious incidents related to certain prescription insomnia medicines
Rare, complex sleep behaviors specifically associated with use of eszopiclone (Lunesta), zaleplon (Sonata) and zolpidem (Ambien, Ambien CR, Edluar, Intermezzo, Zolpimist)
- Serious injuries because of sleep behaviors, including sleepwalking, sleep driving, and engaging in other activities while not fully awake (e.g. using a stove)
- Have also resulted in deaths
Labeling updates
- Boxed Warning –most prominent warning
- Contraindication-strongest warning
- Avoid use in patients who have previously experienced an episode of complex sleep behavior with eszopiclone, zaleplon, and zolpidem
Image credit: FDA