News & Vews: Farewell Commissioner Gottlieb, Patient participation in cancer clinical trials, CDRH reorganization, Request to Connect for patients, Implanted Brain-Computer Interface devices, Safe Use alerts
 Farewell Commissioner Gottlieb
Farewell Commissioner Gottlieb
Sincere gratitude for your leadership and exemplary work during your current FDA tenure. You have been an inspiration. EXIT INTERVIEW
EXIT INTERVIEW

New steps to broaden patient participation in cancer clinical trials
FDA issued new recommendations for broadening cancer trial eligibility criteria
- More representative of patient population
- Maximize the generalizability of the trial results
- Maximize understanding of therapy’s benefit-risk profile across the patient population likely to receive the drug in clinical practice
Broadening eligibility criteria to include: Pediatric patients, Patients with HIV, Hepatitis B and Hepatitis C Virus infections, Patients with brain metastases, organ dysfunction and prior or concurrent malignancies
New Guidances
- Cancer Clinical Trial Eligibility Criteria: Minimum Age for Pediatric Patients; Draft Guidance for Industry
- Cancer Clinical Trial Eligibility Criteria: Patients with HIV, Hepatitis B Virus or Hepatitis C Virus Infections; Draft Guidance for Industry
- Cancer Clinical Trial Eligibility Criteria: Patients with Organ Dysfunction or Prior or Concurrent Malignancies; Draft Guidance for Industry
- Cancer Clinical Trial Eligibility Criteria: Brain Metastases; Draft Guidance for Industry
- Considerations for the Inclusion of Adolescent Patients in Adult Oncology Clinical Trials; Guidance for Industry
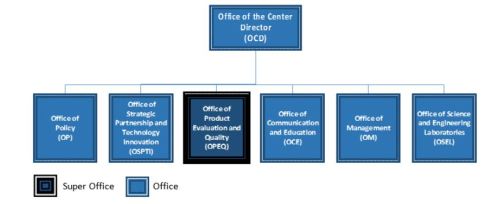
Reorganization of CDRH
CDRH reorganization to implement efficiencies to integrate CDRH’s premarket and postmarket program functions
- Reviewers, compliance officers and other experts would work in teams responsible for device oversight throughout the product’s development and commercialization
- More integrated approach to device safety throughout the Total Product Life Cycle (TPLC) – already in pilot testing
- Timeline: March 18, 2019 (begin) – September 30, 2019 (full implementation)
New Offices
- Office of Product Evaluation and Quality (OPEQ) : Combines Office of Compliance, Office of Device Evaluation, Office of Surveillance and Biometrics, and Office of In Vitro Diagnostics
- Office of Policy – Two teams, the Guidance, Legislation and Special Projects Team and the Regulatory Documents and Special Projects Team
- Office of Strategic Partnerships and Technological Innovation – Combines Science & Strategic Partnerships, Digital Health, Health Informatics and Innovation teams

“Request to Connect” – A New Way for Patients to Connect with FDA
- Gives patients and caregivers a single entry point to the Agency for questions and meeting requests
- Co-developed by Patient Affairs Staff and the medical product centers
- Will route inquiries to appropriate medical product center or office for responses in effective and efficient manner
- Opportunity for FDA to better understand patient perspective and advance science of patient input
Implanted Brain-Computer Interface (BCI) Devices for Patients with
Paralysis or Amputation – Non-clinical Testing and Clinical
Considerations
Implanted BCI devices increase ability to interact with environment and provide independence to patients with paralysis or amputation
- Interface with nervous system to restore motor and/or sensory capabilities
- Recommendations for non-clinical testing and study design considerations for IDE feasibility and pivotal clinical studies
Non-Clinical Bench Testing Considerations: Risk analysis, Electrodes, Leads and Connectors, Implanted casing and electronics, Output simulation measurements, Output simulation safety, Programmers/control unit, Radiofrequency transmitter and receiver, System level testing, factors for determining and design of animal studies
Clinical Study Considerations: Patient population, Home-Use, Duration and follow-up schedule, Inclusion/Exclusion criteria, Demographics, Treatment parameters, Endpoints including patient input (patient engagement. patient preference information, patient reported outcome measures)

SAFE USE ALERTS: Surgical Staplers and Staples, Contact Lenses
Surgical Staplers and Staples:
- Increased number of adverse events reported to the FDA since 2011: 366 deaths, > 9,000 serious injuries, > 32,000 malfunctions
- FDA Actions:
- Letter to Healthcare Providers
- Issue draft guidance on recommendations to manufacturers
- Hold public meeting of General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee to reclassify as Class II (from Class I)
- Require premarket notification and establish mandatory special controls to help mitigate known risks
Contact lenses
- Risk of several serious conditions including eye infections and corneal ulcers
- Can develop very quickly, be serious and can cause blindness
- Video on safe and effective use
Image credits: FDA
