News & Views: Patients First, Least Burdensome Provisions, Drug Supply Chain security, 510(K) exemptions
 FDA’s Patient Affairs Staff (PAS)- FDA Puts Patients First
FDA’s Patient Affairs Staff (PAS)- FDA Puts Patients First
PAS works with patients, caregivers, and advocates in 2019
- Understanding patient experience and incorporating patient feedback into FDA’s work
PAS launched several initiatives to support and connect with patients
- Patients Matter video series
- FDA/NORD Rare Disease Listening Session pilot
- Patient Engagement Collaborative (PEC)
- FDA Voices post

The Least Burdensome Provisions: Concept and Principles
Final guidance on use of least burdensome approach to medical device regulation
- Support timely patient access to high quality, safe and effective medical devices
- Remove or reduce unnecessary burdens throughout total product lifecycle
- Maintaining statutory requirements for clearance and approval
- Principles are based on sound science, intent of the law, use of alternative approaches, efficient use of resources to effectively address regulatory issues
Guiding principles
- FDA to request minimum information necessary to adequately address regulatory question or issue at hand
- Industry should submit material, including premarket submissions, that are least burdensome for FDA to review
- FDA to use most efficient means to resolve regulatory questions and issues
- Right information provided at right time (e.g., just-in-time data collection) to address right questions
- Regulatory approaches designed to fit technology, taking into account unique innovation cycles, evidence generation needs, timely patient access
- FDA to leverage outside US data to the extent appropriate and feasible
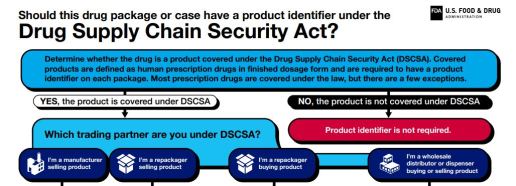
Improving security of Drug Supply Chain through innovations that improve tracking and tracing of medicines

Intent to Exempt Certain Unclassified Medical Devices from Premarket Notification Requirements
Intent to exempt certain unclassified medical devices from premarket notification requirements
- Devices are sufficiently well understood
- Do not require premarket notification (510(k)) to assure their safety and effectiveness.
Device categories
- Ear, Nose, and Throat
- Gastroenterology-Urology
- General and Plastic Surgical
- Neurological
- Obstetrical and Gynecological Devices
- Physical Medicine Devices
Image credit: FDA