News & Views: Duodenoscope concerns, Biomarker qualification framework, Device classification procedures, Data integrity, NASH guidance, Non-device software report, To eat or not to eat?

Interim Results of Duodenoscope Reprocessing Studies Conducted in Real-World Settings: FDA Safety Communication
FDA ordered all U.S. duodenoscope manufacturers (Olympus, Fujifilm, Pentax) to conduct 2 postmarket surveillance studies on whether health care facilities were able to properly clean and disinfect devices
- Study to sample and culture reprocessed duodenoscopes
- Study to nassess how effectively the trained hospital staff follow the manufacturer reprocessing instructions (human factors)
- At least 10% of the samples have been collected assuming <0.4% contamination rate
Interim results indicate higher-than-expected contamination rates after reprocessing
- Up to 3% samples testing positive for enough low concern organisms
- Up to 3% of samples testing positive for high concern organisms- E. coli, Pseudomonas aeruginosa
- Root cause analyses are underway; final results in 2019
Safety communication to hospitals and health care providers
- Following the manufacturer’s reprocessing and maintenance instructions not sufficient to avoid all infections
- FDA been working with developers on new product designs, including disposable components
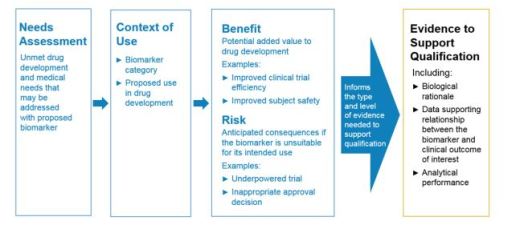
Biomarker Qualification: Evidentiary Framework
Evidentiary framework to support biomarker qualification with recommended components
- Type and level of evidence
- Broadly applicable regardless of the type of biomarker or context of use (COU)
- Qualified biomarker can be used across multiple drug development programs
Framework consists of
- Drug development need
- Defining the COU
- Potential benefits if the biomarker is qualified for use
- Potential risks of biomarker in drug development program
Biomarker validation

Medical Device Classification Procedures: Incorporating Food and Drug Administration Safety and Innovation Act Procedures
Scope
- Medical device classification procedures for certain devices and requiring premarket approval applications for preamendments to Class III devices by using administrative orders, rather than rulemaking
- Do not affect the classifications of previously cleared or approved devices
Principle benefits
- Reduction in regulatory and economic burden and decreased timelines by eliminating paperwork filing requirements
- Enhanced consistency and uniformity across reclassification proceedings
- Cost and time savings will accrue to both medical device manufacturers and to Agency.

Data Integrity and Compliance With Drug CGMP
Clarify role of data integrity in current good manufacturing practice (CGMP) for drugs
- All data expected to be reliable and accurate
- Flexible and risk-based strategies to prevent and detect data integrity issues
- Consider design, operation, monitoring based on risk to patient, process, product
- Management’s involvement essential in preventing and correcting conditions that lead to data integrity problems.
- Create a quality culture with data integrity as core value
Q & As on broad range of topics provided

Noncirrhotic Nonalcoholic Steatohepatitis With Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry
Guidance for clinical development of drugs for treatment of noncirrhotic nonalcoholic steatohepatitis (NASH) with liver fibrosis
- Necessary components of drug development program
Overview:
- General Considerations
- Phase 2 Development Considerations: Early and Late trials
- Phase 3 Development Considerations: Inclusion / Exclusion criteria, trial design and efficacy endpoints, safety considerations, pediatric considerations

Report on Non-Device Software Functions
Certain software functions excluded from definition of device under section 201(h) FD&C Act (21 U.S.C. 321(h))
- Administrative support of a health care facility
- Maintaining or encouraging a healthy lifestyle and unrelated to the diagnosis, cure, mitigation, prevention, or treatment of disease or condition
- Serving as electronic patient records; not intended to interpret or analyze
- Transferring, storing, converting formats, displaying data
- Certain types of clinical decision support to a health care provider unless interpreting or analyzing
Report based on information from: DHHS offices, representatives of patients, consumers, health care providers, startup companies, health plans or other third-party payers, venture capital investors, information technology
Analysis based on: Impact to patient safety, Benefits and risks to health, Best practices to promote safety, education, and competency
Findings: More benefits than risks to patient safety and health related to these software functions
Details best practices: Implementation, training techniques, and use, which could promote safety, education, and competency related to these software functions
Image credit: FDA