
Framework for Real-World Evidence Program
- Already approved under section 505(c) of the FD&C Act
- Adding or modifying indication, adding new population, adding comparative effectiveness or safety information
- Help satisfy postapproval study requirements
- Does not apply to medical devices
Three-part approach for assessing use of real world data (RWD) to generate RWE
- Are the RWD fit for use?
- Can study design used to generate RWE provide adequate relevant scientific evidence?
- Does study conducted meet FDA regulatory requirements for study monitoring and data collection?
Women’s Health Research Roadmap
FDA Office of Women’s Health (OWH) research priority areas to advance the science of women’s health
- Advance Safety and Efficacy of FDA-regulated products used by women
- Improve Clinical Study Design and Analyses to better identify and evaluate possible sex differences
- Novel Modeling and Simulation Approaches to aid in regulatory evaluation
- Advances in Biomarker Science to identify, evaluate, qualify clinical and non-clinical biomarkers and surrogate endpoints
- Expand Data Sources and Analysis to identify, develop, evaluate data sources to assess postmarket toxicity or safety and effectiveness
- Improve Health Communications to develop, evaluate, use tools to make informed health-related decisions
- Emerging Technologies for identification of sex differences to use of emerging technologies

New Steps to Advance Clinical Testing to Deliver New Cures

FDA Recognition of Public Human Genetic Variant Databases
Genetic variant database collect, organize, publicly document evidence supporting links between human genetic variant and disease
- Better understanding relationships between genotypes and diseases can aid in the diagnosis and treatment of individuals with genetic conditions
- Mechanism for test developers to leverage publicly accessible databases to support regulatory review of genetic and genomic tests
Clinical Genome Resource (ClinGen) consortium’s ClinGen Expert Curated Human Genetic Data is first FDA-recognized publicly-available genetic variants database

Improvements to the De Novo pathway for novel medical devices to advance safe, effective, and innovative treatments for patients
Establish procedures and criteria for medical device De Novo classification process
- For new medical devices with no legally marketed predicates
- Vehicle for establishing new predicates reflecting modern standards for performance and safety
- Make pathway significantly more efficient and transparent
- Expecting more use of pathway for new devices
De Novo request granted
- new device is legally marketed and in compliance with regulatory controls
- new classification regulation established
- new device serves as predicate device for 510(k) submissions of same type devices
- Federal Register notification of new classification regulation, new special controls
- posting of granting order and decision summary.
De Novo request decline
- general/special controls insufficient to provide reasonable assurance of safety and effectiveness
- data insufficient to determine whether controls can provide reasonable assurance of safety and effectiveness of the device
- probable benefits do not outweigh probable risks
- device remains in class III and may not be legally marketed

Collaborative Community Toolkit
Collaborative community is continuing forum with private- and public-sector members, including FDA, work together on medical device challenges
- Challenges ill-defined or no consensus
- Challenges and outcomes are complex
- Partners are interrelated
- Incremental or unilateral efforts to address challenge have been ineffective
- Partners seek to optimize efforts, including preventing duplication of efforts
- Better outcomes with integrating different perspectives, experiences, resources, expertise
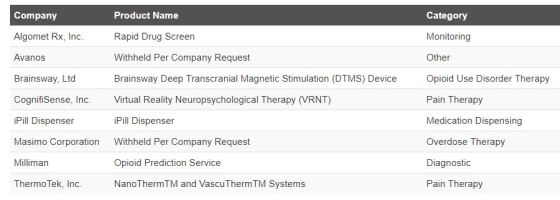
Selections for FDA Innovation Challenge for Devices to Prevent and Treat Opioid Use Disorder
Over 250 applications received for Opioid Use Disorder (OUD) challenge; selections include
- prediction of risk of OUD
- detection of opioid overdose
- dispensing medication
- providing pain treatment alternatives to opioids
Selected applicants receive Breakthrough Device designation

Steps to advance safety and accuracy of blood glucose monitors
Two revised draft guidances regarding blood glucose monitors used in health care and home settings
- Based stakeholder feedback requesting more clarification on design considerations and recommended standards
- To improve development of new blood glucose meters based on feedback from both patients and health care providers especially on usability of glucose monitors
Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use
Self-Monitoring Blood Glucose Test Systems for Over-the-Counter Use

Patient Engagement Advisory Committee (PEAC)
PEAC held on Nov 15, 2018, to discuss topic “Connected and Empowered Patients: e-Platforms Potentially Expanding the Definition of Scientific Evidence”
- Leveraging patient-generated health data, such as social media, sensors, patient-driven registries, to better engage patients
- Social media and other web platform for virtual patient communities
- Resource for postmarket study design, performing surveillance for adverse events, issuing recalls, communicating signals to public
FDA needs to take following steps
- Standardize how data is collected, used and applied
- Ensure language communicated to patients be clear and understandable
- Ensure data collected is de-identified
- Ensure a high level of integrity with the data collected
Image credits: FDA

