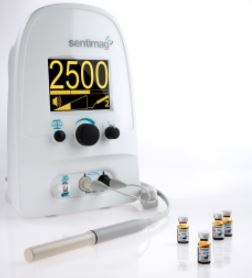
Magtrace and Sentimag Magnetic Localization System
Endomagnetics Inc.
INDICATION FOR USE: To assist in localizing lymph nodes draining a tumor
site, as part of a sentinel lymph node biopsy procedure, in patients with breast cancer undergoing a mastectomy. Magtrace™ is intended and calibrated for use ONLY with the Sentimag® system.
ADDRESSING UNMET NEED:
- Sentinel lymph node biopsies are crucial for determining whether a patient’s breast cancer has spread and helping the provider determine the most appropriate course of treatment
- System offers patients undergoing mastectomy an option for their sentinel lymph biopsy procedure that does not require the injection of radioactive materials
SYSTEM DESCRIPTION:
- Sensitive magnetic sensing probe and base unit designed to detect small amounts of Magtrace, the magnetic tracer drug that is injected into breast tissue
- Magtrace particles travel to lymph nodes, become physically trapped in them, facilitating magnetic detection of the lymph nodes
- Following injection of Magtrace, the Sentimag probe is applied to the patients’ skin in areas closest to the tumor site containing the lymph nodes
- Sensing of the magnetic particles is indicated by changes in audio and visual alerts from the base unit, enabling the surgeon to move the hand-held probe around the area of the lymph nodes, and locate the sentinel lymph node or nodes (if there are more than one)
- Surgeon makes a small incision and removes the node, which is checked by a pathologist for the presence of cancer cell
- Negative result: Suggests cancer has not spread to nearby lymph nodes
- Positive result: May indicate cancer present in sentinel lymph node, nearby lymph nodes and, possibly, other organs
- Help determine stage of cancer and develop appropriate treatment plan
EFFECTIVENESS AND SAFETY:
- Trial of 147 patients with breast cancer, n=147, Sentimag System vs. control methodin sentinel lymph node detection ratw
- 94.3% for Sentimag System vs. 93.5% for control
- Overall, 98.0% same detection rate with both Sentimag System and control
- Most common adverse event: Breast discoloration, cardiac disorder (bradycardia) and potential allergic reaction to the magnetic materials
- Contraindication: Hypersensitivity to iron oxide or dextran compounds
REGULATORY PATHWAY: PMA, Combination Product
- Product Code: PUV
- Classification: III
- Classification Name: Lymph Node Location System during Sentinel Biopsy Procedure
- Coordinated, cross-agency approach- Clinical review conducted by CDRH in consultation with CDER and with support from Oncology Center of Excellence. All other aspects of review and final product approval by CDRH

Surpass Streamline Flow Diverter
Stryker
INDICATION FOR USE: In the endovascular treatment of patients (18 years of age and older) with unruptured large or giant saccular wide-neck (neck width ≥ 4 mm or dome-to-neck ratio < 2) or fusiform intracranial aneurysms in the internal carotid artery from the petrous segment to the terminus arising from a parent vessel with a diameter ≥ 2.5 mm and ≤ 5.3 mm.
DEVICE DESCRIPTION:
- Self-expandable braided device preloaded into a delivery system. Each device is shipped sterile and labeled for single use only
- Consists of the following major components: Surpass Flow Diverter (Implant), Delivery Catheter, Pusher
EFFECTIVENESS AND SAFETY:
- Multi-center, prospective, non-randomized clinical study., n=180, follow-up at discharge, 30 days, 6 months, and 12-months post-procedure
- Primary Safety endpoint: % experiencing neurologic death or major ipsilateral stroke through 12-months post-procedure
- Major Effectiveness endpoint: % with complete (100%) occlusion (Raymond-Roy Class I) of the treated intracranial aneurysm without clinically significant stenosis
- 62.8% achieved complete occlusion of their intracranial aneurysm within 1year post-procedure without re-treatment or clinically significant in-stent stenosis
REGULATORY PATHWAY: PMA
- Product Code: OUT
- Classification: III
- Generic Name: Intracranial Aneurysm Flow Diverter
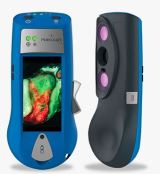
MolecuLight i:X
MolecuLight Inc.
INDICATION FOR USE: Handheld imaging tool that allows clinicians diagnosing and treating skin wounds, at the point of care, to:
- view and digitally record images of a wound, and
- view and digitally record images of fluorescence emitted from a wound when exposed to an excitation light
For prescription use only.
GENERIC DEVICE TYPE: Wound autofluorescence imaging device
Tool to view autofluorescence images from skin wounds that are exposed to an excitation light. The device is not intended to provide quantitative or diagnostic information
REGULATORY PATHWAY: De Novo request
- Classification: I
- Product Code: QCR
- Regulation Number: 21 CFR 878.4165
- Regulation Name: Wound autofluorescence imaging device
IDENTIFIED RISKS: Electrical/mechanical/thermal, electromagnetic compatibility (EMC) and optical safety of the device, and the error in fluorescence detection from the wound.
- No special controls
POTELIGEO (mogamulizumab-kpkc) injection
Kyowa Hakko Kirin
INDICATION FOR USE: Treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy
ADDRESSING UNMET NEED:
- Mycosis fungoides and Sézary syndrome are rare, hard-to-treat types of non-Hodgkin lymphoma
- Fills an unmet medical need for these patients
MECHANISM OF ACTION: CC chemokine receptor type 4 (CCR4)-directed monoclonal antibody; CCR4 present in some cancern cells
EFFICACY:
- Clinical trial, n=372 patients with relapsed MF or SS, Poteligeo vs. vorinostat
- Progression-free survival: Median 7.6 months vs. median 3.1 months
SAFETY:
- Most common side effects: Rash, infusion-related reactions, fatigue, diarrhea, musculoskeletal pain and upper respiratory tract infection
- Serious warnings: Risk of dermatologic toxicity, infusion reactions, infections, autoimmune problems, complications of stem cell transplantation that uses donor stem cells (allogeneic) after treatment with the drug
REGULATORY PATHWAY: BLA
- Priority Review and Breakthrough Therapy designation, Orphan Drug designation
- Postmarketing Requirements: Characterize complications after allogeneic hematopoietic stem cell transplantation
- Exempt from pediatric assessments
Image credit: Endomagnetics, Stryker, MolecuLight Inc., Kyowa Hakko Kirin