Biosimilars Action Plan (BAP)
Focus on four key areas
- Improving efficiency of biosimilar and interchangeable product development and approval process
- Maximizing scientific and regulatory clarity for biosimilar product development community;
- Developing effective communications to improve understanding of biosimilars among patients, clinicians, and payors
- Supporting market competition by reducing gaming of FDA requirements or other attempts to unfairly delay competition
Actions will help create competitive market, provide incentives for sponsors
Commissioner Gottlieb’s remarks “Dynamic Regulation: Key to Maintaining Balance Between Biosimilars Innovation and Competition” at Brookings Institution about BAP

CDER Conversation: Treatment for Opioid Use Disorder
Medication assisted treatment (MAT) is key to combating opioid use disorder (OUD)
- Evidence-based, FDA approved medication (methadone, buprenorphine, naltrexone) combined with counseling and psychosocial support
- Decrease cravings, relieve withdrawal symptoms; none cause euphoria or “high”
Use Information
- Can be administered through different routes depending on specific drug; however, many clinicians are not assessing and treating patients with OUD
- Potential for drug-drug interactions
- Recommended for pregnant women with substance use disorder
- SAMHSA’s treatment locator service can be helpful in finding a nearby treatment program
 Protecting and Promoting Public Health: Advancing the FDA’s Medical Countermeasures Mission
Protecting and Promoting Public Health: Advancing the FDA’s Medical Countermeasures Mission
Role in national security by facilitating development and availability of safe and effective medical countermeasures
- Animal Rule: Efficacy data obtained solely from animals when studies in humans are not ethical or feasible
- New guidances: eg smallpox
- Emergency Use Authorizations: Facilitating availability and use of MCMs needed during public health emergencies
- FDA and DoD joint program for development of medical oroducts intended to save American military personnel
- FDA’s Medical Countermeasures Initiative (MCMi) established in 2010

New efforts to empower consumers by advancing access to nonprescription drugs
Increase access to broader selection of nonprescription drug products empowering consumers to self-treat common conditions and potentially some chronic conditions
- lower costs for health care system overall
- provide greater efficiency and empowerment for consumers
- access to resources to help patients determine if medicine is right for them
Use of innovative technologies
- Mobile Medical App with a set of questions to help someone determine (self-select) appropriateness of drug for them
- Sponsors could develop approaches for self-selection and accurate use of their prescription product in the nonprescription setting
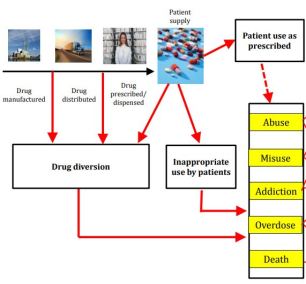 Agency’s efforts to encourage the development of and broaden access to generic versions of opioid analgesics that are formulated to deter abuse
Agency’s efforts to encourage the development of and broaden access to generic versions of opioid analgesics that are formulated to deter abuse
Encouraging development of opioids with abuse-deterrent formulations (ADFs) intended to make certain types of abuse, e.g. crushing, dissolving, more difficult or less rewarding
- New 43 product-specific guidances related to the development of generic drug products
- In vivo and in vitro study considerations for abuse deterrence evaluations
- Final guidance on development of generic versions of approved ADF opioids
Image credits: FDA

