EPIDIOLEX (cannabidiol) oral solution
GW Pharmaceuticals
INDICATION: Treatment of seizures associated with Lennox-Gastaut syndrome (LGS) or Dravet syndrome (DS) in patients 2 years of age and older
ADDRESSING UNMET NEED:
- Treatment of rare genetic conditions in pediatric population
- First botanical marijuana product to be FDA approved
MECHANISM OF ACTION: Precise mechanisms of anticonvulsant effect unknown; does not appear to exert its anticonvulsant effects through interaction with cannabinoid receptors
EFFICACY:
- Three randomized, double-blind, placebo-controlled clinical trials, n=516 patients with either Lennox-Gastaut syndrome or Dravet syndrome, Epidiolex + other medications vs. placebo, 14-week treatment period
- Primary efficacy measure: %change from baseline in the frequency (per 28
days) of drop seizures (atonic, tonic, or tonic-clonic seizures) - Significant decrease in seizures vs placebo; p≤0.01
SAFETY:
- Most serious risks: Thoughts about suicide, attempts to commit suicide, feelings of agitation, new or worsening depression, aggression and panic attacks
- Also caused liver injury, generally mild, but raising the possibility of rare, but more severe injury
- Most common side effects: Sleepiness, sedation and lethargy; elevated liver enzymes; decreased appetite; diarrhea; rash; fatigue, malaise and weakness; insomnia, sleep disorder and poor quality sleep; and infections
- Must be dispensed with a patient Medication Guide
REGULATORY PATHWAY:
FDA
- Orphan designation, Fast Track, Priority Review, Priority Review Voucher
- Postmarketing requirements: Embryofetal development study, juvenile toxicology, carcinogenicity, potential for chronic liver injury, pregnancy outcomes study, risk of drug-drug interactions or QT interval prolongation.
DEA
- Currently controlled in Schedule I under the Controlled Substances Act
- FDA scheduling recommendation has been transmitted to DEA
- Final DEA scheduling decision pending
ZEPHYR Endobronchial Valve (Zephyr Valve)
Pulmonx
INTENDED USE: Treat breathing difficulty associated with severe emphysema
ADDRESSING UNMET NEED: Less invasive treatment that expands the options available to emphysema patients
DEVICE DESCRIPTION:
- Flexible bronchoscope to place Zephyr Valves, similar in size to pencil erasers, into diseased areas of lung airways
- Device design intended to prevent air from entering damaged parts of lung and allow trapped air and fluids to escape
- During inhalation, valves close, preventing air from entering damaged part of lung
- During exhalation, valves open, letting out trapped air, which is intended to relieve pressure
EFFECTIVENESS:
- Study, n=190 emphysema patients, Zephyr Valves + medical management (medications and pulmonary rehabilitation) vs. Control group received medical management only, one year treatment
- Primary endpoint: % with at least a 15% improvement in pulmonary function scores (the volume of air that can forcibly be blown out in one second after full inhalation)
- 47.7 % with Zephyr Valves vs. 16.8% in control group
SAFETY:
- Adverse events: Death, air leak (pneumothorax), pneumonia, worsening of emphysema, coughing up blood, shortness of breath and chest pain
- Contraindication: Active lung infections; those who are allergic to nitinol, nickel, titanium or silicone; active smokers and those who are not able to tolerate the bronchoscopic procedure
REGULATORY PATHWAY: PMA
- Breakthrough designation
Ellipsys Vascular Access System
Avenu Medical
INDICATION FOR USE: Creation of a proximal radial artery to perforating vein anastomosis via a retrograde venous access approach in patients with a minimum vessel diameter of 2.0mm and less than 1.5mm of separation between the artery and vein at the fistula creation site who have chronic kidney disease requiring dialysis

EVERLINQ endoAVF System
TVA Medical
INDICATION OF USE: Creation of an arteriovenous fistula (AVF) using the ulnar artery and ulnar vein in patients with minimum artery and vein diameters of 2.0 mm and less than 2.0 mm separation between the artery and vein at the fistula creation site who have chronic kidney disease and need hemodialysis
………………..
ADDRESSING UNMET NEED: Additional, less-invasive vascular access options for patients who will require hemodialysis
DEVICE TYPE: Percutaneous catheter for creation of an arteriovenous fistula for hemodialysis access
- Single use percutaneous catheter system that creates an arteriovenous fistula (AVF) in the arm of patients with chronic kidney disease who need hemodialysis
CLINICAL PERFORMANCE:
- Safely deliver, deploy, and remove the device
- Create an arteriovenous fistula
- Attain blood flow rate and diameter suitable for hemodialysis
- Use fistula for vascular access for hemodialysis
- Patency of the fistula
RISKS:
- Unintended vascular or tissue injury
- Adverse hemodynamic effects
- Failure to create a durable fistula that is usable for hemodialysis
- Use of the device adversely impacts future vascular access sites
- Adverse tissue reaction
- Infection
- Electrical malfunction or interference leading to electrical shock, device failure, or inappropriate activation
- Software malfunction leading to device failure or inappropriate activation
REGULATORY PATHWAY: De Novo request
- Regulation Number: 21 CFR 870.1252
- Regulation Name: Percutaneous catheter for creation of an arteriovenous fistula for hemodialysis access
- Regulatory Class: Class II
- Product Code: PQK
CLASSIFICATION ORDER (Ellipsys)
CLASSIFICATION ORDER (everlinQ)
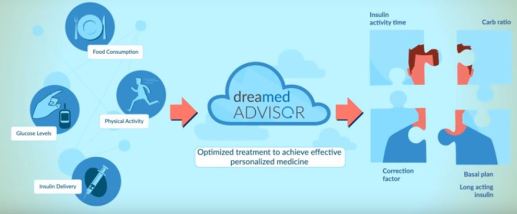
DreaMed Advisor Pro decision-support software
DreaMed Diabetes, Ltd
INDICATION FOR USE: Decision-support software intended for assisting healthcare professionals in the management of patients with Type 1 diabetes who:
- use insulin pumps as their insulin delivery therapy
- monitor their glucose levels using either of the following: CGM, or o CGM and self-management blood glucose meter
- Are above the age of 6 and under 65 years old
- Use rapid acting U-100 insulin analogs in their pump
Use by healthcare professionals when analyzing continuous glucose monitoring (CGM), self-monitoring blood glucose (SMBG) and pump data to generate recommendations for optimizing a patient’s insulin pump settings for basal rate, carbohydrate ratio (CR), and correction factor (CF); without considering the full clinical status of a particular patient.
DreaMed Advisor Pro does not replace clinical judgment
DEVICE TYPE: Insulin Therapy Adjustment Device
- Incorporate biological inputs, including glucose measurement data from a CGM to recommend insulin therapy adjustments as an aid in optimizing insulin therapy regimens for patients with diabetes mellitus
DESIGN VERIFICATION & VALIDATION:
- Required data inputs, including timeframe over which data inputs must be collected and number of data points required
- Types of device outputs and insulin therapy adjustment recommendations, including how the recommendations are generated
- Clinical validity of the device outputs and insulin therapy recommendations
- Input data specifications, including accuracy requirements for CGM and other devices generating data inputs
- Clinical justification for each specification
- Ensure secure and reliable means of data transmission to and from device, data integrity checks, accuracy checks, reliability checks, security measures
- Users can understand and appropriately interpret recommendations
- Mitigation strategy to minimize dosing recommendation errors
RISKS:
- Erroneous or extreme changes in insulin dosing recommendations may cause hypoglycemia or hyperglycemia
- Incorrect interpretation of results may lead to inappropriate clinical decision making
- Incorrect understanding of appropriate device use may lead to inappropriate treatment decisions
- Patient harm due to insecure transmission of data
- Data corruption may lead to inappropriate treatment recommendations
REGULATORY PATHWAY: De Novo request
- 21 CFR 862.1358
- Regulation Name: Insulin Therapy Adjustment Device
- Regulatory Class: Class II
- Product Code: QCC

EVERSENSE Continuous Glucose Monitoring (CGM) system
Senseonics
INDICATION FOR USE: For continually measuring glucose levels in adults (18 years and older) with diabetes for up to 90 days.The system is intended to:
- Provide real-time glucose readings
- Provide glucose trend information
- Provide alerts for the detection and prediction of episodes of low blood glucose (hypoglycemia) and high blood glucose (hyperglycemia)
The system is a prescription device. Historical data from the system can be interpreted to aid in providing therapy adjustments. These adjustments should be based on patterns seen over time.The system is indicated for use as an adjunctive device to complement, not replace, information obtained from standard home blood glucose monitoring devices.
ADDRESSING UNMET NEED:
- More seamless digital system giving patients ability to effectively manage diabetes
- Modern regulatory approach for products that’s carefully adapted to the unique characteristics of these opportunities
DESCRIPTION: Continuous Glucose Monitor, Implanted, Adjunctive Use
EFFECTIVENESS & SAFETY:
- Clinical study, n=1254 diabetes patients, comparing readings by Eversense CGM vs. laboratory-based glucose analyzer
- Safety based on procedure used to implant; <1% experiences serious adverse event
- Potential adverse effects: Related to insertion, removal and wear of the sensor include allergic reaction to adhesives, bleeding, bruising, infection, pain or discomfort, scarring or skin discoloration, sensor fracture during removal, skin inflammation, thinning, discoloration or redness
- Other risks: Hypoglycemia or hyperglycemia in cases where information provided by the device is inaccurate or where alerts are missed
REGULATORY PATHWAY: PMA
- Advisory Committee meeting: 8 to 0 vote, committee recommended that benefits outweigh the risks for patients with diabetes
Image credit: GW, Pulmonx, Avenu, TVA, DreaMed, Senseonics