 Acuvue Oasys Contact Lenses with Transitions Light Intelligent Technology
Acuvue Oasys Contact Lenses with Transitions Light Intelligent Technology
Johnson & Johnson Vision care
USE: Soft Contact lenses that automatically darkens the lens when exposed to bright light. Indicated for daily use to correct the vision of people with non-diseased eyes who are nearsighted (myopia) or farsighted (hyperopia).
ADDRESSING UNMET NEED: First contact lens to incorporate the same technology that is used in eyeglasses that automatically darken in the sun
DESCRIPTION:
- Contains photochromic additive that adapts the amount of visible light filtered to the eye based on the amount of UV light to which they are exposed
- Results in slightly darkened lenses in bright sunlight that automatically return to a regular tint when exposed to normal or dark lighting conditions.
SAFETY AND EFFECTIVENESS:
- Clinical study of 24 patients that evaluated daytime and nighttime driving performance while wearing the contact lenses
- No evidence of concerns with either driving performance or vision while wearing the lenses
- May cause inflammation or infection in or around the eye or eyelids
REGULTORY PATHWAY: 510(k)
- Classification: II
- Regulation No. : 886.5925
- Classification Product Code: LPL
- Subsequent Product Code: MVN
REIMBURSEMENT
- Medicare provides limited coverage for contact lenses under Medicare Vision Services
- Acuvue Brand covered by private payors
IDx-DR Retinal diagnostic software device
IDx LLC
INDICATION FOR USE: For use by health care providers to automatically detect more than mild diabetic retinopathy (mtmDR) in adults diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy. IDx-DR is indicated for use with the Topcon NW400.
ADDRESSING UNMET NEED:
- Diabetic retinopathy is the most common cause of vision loss among the more than 30 million Americans living with diabetes
- Leading cause of vision impairment and blindness among working-age adults
- First medical device to use Artificial Intelligence (AI) to detect greater than a mild level of the eye disease diabetic retinopathy in adults who have diabetes
DESCRIPTION:
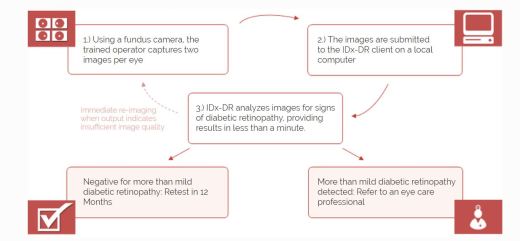
- Software program using AI to analyze eye images taken with retinal camera, Topcon NW400
- Doctor uploads digital images of retinas to cloud server with IDx-DR software
- Software provides doctor with one of two results
- “more than mild diabetic retinopathy detected: refer to an eye care professional”
- “negative for more than mild diabetic retinopathy; rescreen in 12 months.”
- If positive result – further diagnostic evaluation and possible treatment as soon as possible
GENERIC DEVICE TYPE: Retinal diagnostic software device.
Prescription software device that incorporates an adaptive algorithm to evaluate ophthalmic images for diagnostic screening to identify retinal diseases or conditions.
ACCURACY & PRECISION:
- Clinical study of retinal images, n=900 patients with diabetes, 10 primary care sites
- Correctly identify presence of more than mild diabetic retinopathy: 87.4%
- Correctly identify patients who did not have more than mild diabetic retinopathy: 89.5%
IDENTIFIED RISK & MITIGATION MEASURE:
- False positive results leading to additional unnecessary medical procedures (Diagnostic algorithm failure, Software failure): Clinical performance testing;
Software verification, validation, and hazard analysis; Protocol for technical specification changes - False negative results leading to delay of further evaluation or treatment
(Diagnostic algorithm failure, Software failure): Clinical performance testing
Software verification, validation, and hazard analysis; Protocol for technical specification changes; Labeling - Operator failure to provide images that meet input quality specifications: Labeling,
Training, Human factors validation testing
REGULATORY PATHWAY: De Novo request
- Regulation No.: 21 CFR 886.1100
- Regulation Name: Retinal diagnostic software device
- Regulatory Class: Class II
- Product Code: PIB
REIMBURSEMENT:
- AI diagnostic approach could support CMS’ value-based reimbursement

Organ Care System (OCS) Lung System
TransMedics, Inc.
INDICATION FOR USE: Portable organ perfusion, ventilation, and monitoring medical device indicated for the preservation of standard criteria donor lungs in a near physiologic, ventilated, and perfused state for double lung transplantation.
DESCRIPTION:
- Lung Console: Non-sterile, reusable, portable enclosure housing electronic display and non-sterile mechanical/electrical elements to warm, pump, ventilate, and manage gas content of perfusate
- Lung Perfusion Set (LPS): Sterile, single-use perfusion module, organ chamber and circulatory system to perfuse and ventilate lung, facilitate management of fluids
- OCS™ Lung Solution: High oncotic solution used for ex-vivo flush and perfusion of donor lungs when combined with packed red blood cells (pRBCs)
EFFECTIVENESS AND SAFETY:
- Randomized, controlled, multi-center, international, prospective
clinical trial, OCS™ Lung System vs. current cold storage standard of care (SOC), n=407 - Primary Graft Dysfunction (PGD) grading, including reduced survival and
increased incidence of Bronchiolitis Obliterans Syndrome (BOS) - Patient survival at day 30 post-transplantation and ISHLT PGD3 within 72 hours post-transplantation
- No-inferiority vs. SOC, longer-term (2-year) survival and BOS rates comparable
- Similar lung graft-related serious adverse events (LGRSAEs) through 30 days post-transplantation
REGULATORY PATHWAY: PMA
- Class III, Product Code: QBA
- Priority Review
- Gastroenterology-Urology Devices Panel Meeting: Voted 11-2 that there is reasonable assurance the device is safe, 8-5 that there is reasonable assurance that the device is effective, and 9-4 that the benefits of the device do outweigh the risks
- Post-approval studies : Long-term patient outcomes, OCS Lung Thoracic Organ Perfusion (TOP) PAS Registry
Image credit: J&J, IDX, TransMedic