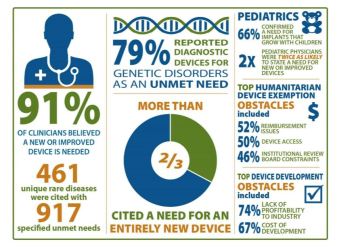
Unmet medical device Needs for patients with rare diseases
FDA, National Center for Advancing Translational Sciences (NCATS)/Office of Rare Diseases Research (ORDR) at NIH sought to better understand medical device needs of patients with rare disease
- Generate meaningful data to inform patients, practitioners, policymakers, and device developers
- Needs, barriers, and incentives
Online survey of four clinician groups
- satisfaction with current devices
- unmet needs for specific rare diseases
- impediments to medical device development
Survey Respondents: 1,342 clinicians
Findings
- Patients with rare diseases face numerous unmet needs
- Device needs of pediatric patients – grow with child, be modified to smaller size, less invasive
- Creating entirely new devices needed vs. modifying/repurposing existing devices
- Limitations included lack of sensitivity/specificity, cumbersome and invasive
- Costs of research, lack of profitability, challenges of conducting trials are challenges

FDA finalizes guidances to accelerate the development of reliable, beneficial next generation sequencing-based tests
Finalized two guidances for efficient development of novel technology that scans DNA to diagnose genetic diseases-next generation sequencing (NGS)
- Recommendations for designing, developing, and validating tests; for continued advancement of individualized, genetic-based medicine
- Modern and flexible framework
- Reliance on clinical evidence from FDA-recognized public databases to support clinical claims e.g. ClinGen
- Recommendations for designing, developing, validating tests to diagnose individuals with suspected genetic diseases
Based on extensive feedback from the public and stakeholders; continuation of creating regulatory efficiencies in the development and review of NGS tests
 FDA Restricts the Sale and Distribution of Essure
FDA Restricts the Sale and Distribution of Essure
Order to restrict the sale and distribution of the Essure device
- Ensure all women provided with adequate risk information so that they can make informed decisions
- Taking this step because some women were not being adequately informed of Essure’s risks before getting the device implanted
- Boxed Warning including perforation of the uterus and/or fallopian tubes, identification of inserts in the abdominal or pelvic cavity, persistent pain, and suspected allergic or hypersensitivity reactions
- FDA closely evaluating new information on the use of Essure; requires additional, meaningful safeguards to ensure women are able to make informed decisions
New Essure labeling
- Restricts sale and distribution to only health care providers and facilities that provide information to patients about the risks and benefits of this device
- Review “Patient-Doctor Discussion Checklist – Acceptance of Risk and Informed Decision Acknowledgement”
- Patient and physician required to sign
- FDA will review and monitor Bayer’s plan to ensure compliance of restriction
Image credit: FDA