Exclusivity and Generic Drugs
Exclusivity and Generic Drugs
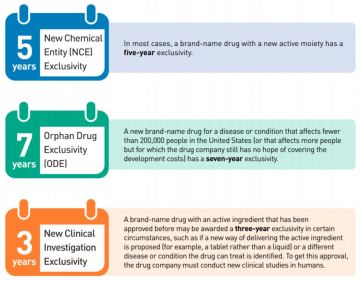
Exclusivity: Period of time when a brand-name drug is protected from generic drug competition
- New Chemical Entity: 5 yr
- Orphan Drug: 7 yr
- New Clinical Investigation: 3 yr
- Pediatric: 6 mo. added on to any other exclusivities or patents
- Antibiotic: 5 yr added on to any other exclusivities
Image credit: FDA