FDA BRIEF: Weeks of November 13 and 20, 2017
 CDRH Approach to Tumor Profiling Next Generation Sequencing (NGS) Tests
CDRH Approach to Tumor Profiling Next Generation Sequencing (NGS) Tests
Marketing authorization of two tumor profiling NGS tests
- Thermo Fisher Scientific’s Oncomine Dx Target Test1 and MSK-IMPACT2
- Real-world application of precision oncology
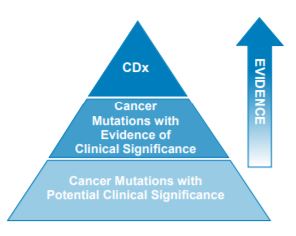
Three-Tiered approach for reporting biomarkers
- Level 1: Companion Diagnostics
- New Level 2: Cancer Mutations with Evidence of Clinical Significance
- Level 3: Cancer Mutations with Potential Clinical Significance
- A Fluid Approach to Reporting within Levels 2 and 3
 Big Day for Regenerative Therapy
Big Day for Regenerative Therapy
Comprehensive policy framework for development and oversight of regenerative medicine products, including novel cellular therapies
- Builds upon the FDA’s existing risk-based regulatory approach
- Proposes efficient, science-based process to ensure safety and effectiveness
- Risk-based framework for enforcement actions against significant safety concerns
Two final guidance documents and Two draft guidance documents
- First guidance provides greater clarity around when cell and tissue-based products
- Second final guidance on application of existing regulatory criteria
- First draft guidance, on simplification and streamlining regulatory requirements for devices
- Second draft guidance on expedited programs
 FDA Workforce and Diversity Plan
FDA Workforce and Diversity Plan
FDA challenged with building and retaining diverse, talented, dedicated workforce
- Building stronger workforce by key process improvements in hiring and retention
- Congress authorized new resources and authorities for talented workforce
FDA Hiring Initiative
- Comprehensive evaluation of our hiring practices and procedures
- Assess current challenges and provide roadmap for future
- Initial Assessment of FDA Hiring and Retention report
- Hiring pilot to modernize and streamline hiring practices; use new IT tools and eliminating unnecessary processes
- Digital and social media tools for modern recruitment and outreach techniques
FDA Diversity and Inclusion Strategic Plan
- Cultivate and promote diverse, inclusive culture
- Promote continuous learning and discussion of diversity and inclusion topics
- Recruit qualified candidates of different backgrounds, experiences, talents
- Leverage every individual’s perspectives, passions, and background; positive
impact on innovation
 Remarks by Commissioner Gottlieb at FDA Office of Criminal Investigation Meeting
Remarks by Commissioner Gottlieb at FDA Office of Criminal Investigation Meeting
Office of Criminal Investigations’s (OCI) nationwide presence
- To advance FDA’s criminal law enforcement operations
- To address criminal wrongdoing involving FDA-regulated products
- Key to stopping dangerous counterfeit, unapproved, misbranded medical products into domestic supply chain
Comprehensive approach to President’s declaration of opioid crisis a public health emergency
- Issuing guidance to encourage development of therapies to treat opioid addiction
- Encouraging widespread use of existing, safe, effective FDA approved therapies to help combat addiction
- Requirement for opioid manufacturers make training available to prescribers, potentially making them mandatory
- Guidances to develop abuse deterrent opioids and non-addictive alternatives in the treatment of pain
- Careful balancing of risks and benefits when making approval decisions or market withdrawals
Dealing with bad actors that see addiction as an opportunity for profit
- Using regulatory authorities and pursuing criminal charges
- Will require manufacturers to follow FDA market withdrawal notices e.g. Endo
- Aggressive steps to identify and disrupt affirmative misconduct
- Criminal enforcement actions in recent months e.g. Insys
- Build cases against individuals who tamper with opioids at pharmacies, hospitals
- Taking further action relating to a botanical substance known as kratom due to risks of abuse, addiction, and death
- Increasing OCI’s Cybercrime Investigations Unit, Strategic Intelligence Unit, and Intelligence Analysis Branch
Steps to promote development of generic versions of opioids formulated to deter abuse
Opioids with abuse-deterrent formulations (ADFs) intended to make abuse, such as crushing, snorting, dissolving, more difficult or less rewarding
- Approved 10 opioid drugs with these properties
- But low uptake- learning curve, low awareness,prescribing uncertainty, price
FDA plans to permanently transition older formulations to ADFs
- Improve access to the newer formulations through generic competitors
- Final guidance on development of generic versions of approved ADF opioids
- Developing appropriate, improved testing methodologies for evaluating abuse deterrence for both brand name and generic opioid drug products
- Flexible, adaptive approach to the evaluation and labeling of ADF opioids
- Determining effectiveness of ADF products in real-world setting
- Better understanding the attitudes and beliefs of health care professionals and those who are prescribed these products
Image credit: FDA