FDA BRIEF: Week of September 18, 2017


Recognized Consensus Standards: Radio-Frequency Wireless Coexistence For Medical Devices And Systems
FDA publication of standards-recognition notice (Recognition List #47) that includes
- American National Standards Institute (ANSI) American National Standard for Evaluation of Wireless Coexistence C63
- Association for the Advancement of Medical Instrumentation (AAMI) Technical Information Report (TIR) 69: Risk management of radio-frequency wireless coexistence for medical devices and systems.
First standard to specifically address wireless coexistence testing to assess ability of wireless equipment to coexist with other wireless equipment in its intended use environment
- For all medical devices that incorporate wireless technology for data transmission, communications or control
- For 510(k), PMA, PDP, HDE, GMP, Design controls
Relevant FDA Guidances
- Radio Frequency Wireless Technology In Medical Devices – Guidance For Industry And Food And Drug Administration Staff, Document Issued On: August 13, 2013
- Information To Support A Claim Of Electromagnetic Compatibility (EMC) Of Electrically-Powered Medical Devices – Guidance For Industry And Food And Drug Administration Staff. Document Issued On: July 11, 2016

In Pursuit of Tardive Dyskinesia: The Breakthrough Designation and Approval of Valbenazine
Tardive dyskinesia – a side effect of taking antipsychotic medicines
- Neurological disorder with repetitive, involuntary movements such as lip smacking or pursing, as well as tongue movements
- Impacts the health and quality of life and is often irreversible
Background
- First-generation antipsychotics and second-generation antipsychotics (“atypicals”) revolutionized psychiatric care but caused tardive dyskinesia
- May be caused by irregular dopamine signaling
- Interest in studying tetrabenzine and valbenazine to treat involuntary movements
A Breakthrough Therapy and Expedited Approval
- Breakthrough Therapy designation based on promising clinical results
- Facilitated discussions between the FDA and valbenazine sponsor (Neurocrine Biosciences)
- Priority Review and drug approval in 8 months after NDA submission

FDA’s continued efforts to promote the safe adoption of Medication-Assisted Treatment for opioid addiction
Medication-assisted treatment (MAT) – the use of medication combined with counseling and behavioral therapies
- Major pillars of federal response to opioid epidemic
However, significant challenges with use of MAT drugs contain methadone or buprenorphine – which are also opioids
- Increased risk of serious side effects when combining MAT drugs with benzodiazepines – often prescribed to treat anxiety, insomnia, or other conditions
- Strengthened labeling for MAT drug buprenorphine to emphasize indefinite treatment and continued treatment as long as it contributes to treatment goals
- Develop treatment plan to closely monitor concomitant use of benzodiazepines and taper use while considering other treatment options

FDA Encourages New Treatments for Sickle Cell Disease
Sickle Cell Disease (SCD)
- “Sickled” or abnormally shaped red blood cells that get stuck in small blood vessels and block the flow of blood and oxygen to major organs
- Cause severe pain, organ damage, or even stroke
- Is chronic and lead to shortened lifespans
Limited Treatment Options
- Approved 1998: Hydroxyurea- for red blood cells to stay round and flexible
- Approved 2017: Endari (L-glutamine oral powder) to reduce acute complications of sudden, severe attacks of pain (sickle cell crises)
Encouraging New Treatment Development
- Working with stakeholders—patients, academics and companies
- “Fast track” designation, to bring new products to market faster
- “Breakthrough therapy” designation if promising preliminary data
- “Orphan status” if intended to treat rare diseases affecting < 200,000 people
- Meeting with patients and learning the patient perspective to integrate into new product development
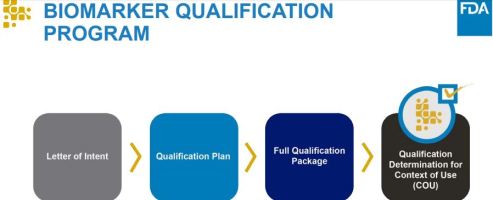
Biomarker Qualification Program
Biomarker Qualification Program (BQP) encourages biomarker development
- For use in drug development, facilitate an efficient review process, make information publicly available
- Qualified biomarker can be used in multiple drug development program without
need to reconfirm suitability of biomarker’s qualified context of use. - Potential to streamline drug development paradigm
Three submission stages
- Letter of Intent
- Qualification Plan
- Full Qualification Package
FDA Engagement throughout the process
- At each milestone, formal written FDA determination letter
- Seek clarification regarding determination letter
- Obtain additional information about qualification process
Image credits: FDA, AAMI, ANSI, Neurocrine
