FDA BRIEF: Week of July 31, 2017


IDHIFA (enasidenib)
Celgene Corp
Abbott RealTime IDH2
Abbott Molecular Inc.
INDICATION: Treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation as detected by an FDA-approved test.
Approved for use with a companion diagnostic, the RealTime IDH2 Assay, to detect specific mutations in the IDH2 gene in patients with AML.
REG PATHWAY: NDA
- Orphan Drug Designation, Priority Review
- Postmaketing requirements: Characterization of long-term safety in AML, Cllinical PK studies for CYP interaction, hepatic impairment
- Companion Diagnostic: Somatic Gene Mutation Detection System
MECHANISM OF ACTION: Inhibitor of the isocitrate dehydrogenase 2 (IDH2) enzyme, decreasing 2-hydroxyglutarate (2-HG) levels, reduced blast counts and increased percentages of mature myeloid cells
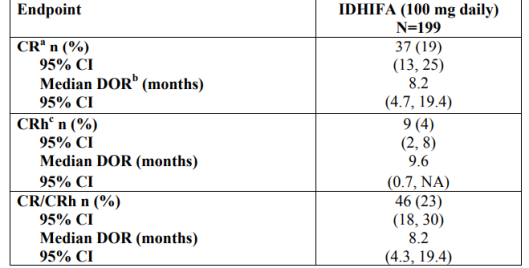
EFFICACY:
- Open-label, single-arm, multicenter, two-cohort clinical trial, n= 199, patients with relapsed or refractory AML and IDH2 mutation (confirmed by RealTime IDH2 Diagnostic)
- Endpoint: Rate of complete response (CR)/complete response with
partial hematologic recovery (CRh), duration of response (DOR), rate of conversion from transfusion dependence to transfusion independence
SAFETY:
- Boxed Warning: Differentiation syndrome-can be fatal if not treated
- Common side effects: Nausea, vomiting, diarrhea, increased levels of bilirubin (substance found in bile) and decreased appetite. Women who are pregnant or breastfeeding should not take Idhifa because it may cause harm to a developing fetus or a newborn baby

VYXEOS (liposome-encapsulated combination of daunorubicin and cytarabine)
Jazz Pharmaceuticals, Inc.
INDICATION: Treatment of adults with newly-diagnosed therapy-related acute
myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC)
ADDRESSING UNMET NEED: First FDA-approved treatment specifically for patients with t-AML or AML-MRC.
REG PATHWAY: NDA, 505(b)(2) pathway
- Orphan designation, Priority review
- Postmarketing requirements: Characterize nature, incidence, severity of infusion-related reactions, determine appropriate in patients with renal impairment
MECHANISM OF ACTION: Daunorubicin has antimitotic and cytotoxic activity and Cytarabine is a cell cycle phase-specific antineoplastic agent
EFFICACY:
- Randomized, multicenter, open-label, active-controlled study, n=309, VYXEOS to a standard combination of cytarabine and daunorubicin (7+3), patients with newly diagnosed t-AML or AML-MRC
- Endpoint: Overall survival from the date of randomization to death
from any cause - 9.6 months vs. 5.9 months, p=0.005
SAFETY:
- Most common adverse reactions: Hemorrhage events, febrile neutropenia, rash, edema, nausea, mucositis, diarrhea, constipation, musculoskeletal pain, fatigue, abdominal pain, dyspnea, headache, cough, decreased appetite, arrhythmia, pneumonia, bacteremia, chills, sleep disorders and vomiting

IMBRUVICA (ibrutinib) capsules
Pharmacyclics LLC
EXPANDED INDICATION: Treatment of adult patients with chronic graft-versus-host disease (cGVHD) after failure of one or more lines of systemic therapy
ADDRESSING UNMET NEED:
- cGVHD is life-threatening; occurs in 30-70% patients who receive HSCT
- This is the first FDA-approved therapy for the treatment of cGVHD
REG PATHWAY: sNDA
- Priority Review, Breakthrough Therapy, Orphan Drug Designation
- Previously approved for certain indications in treating chronic lymphocytic leukemia, Waldenström’s macroglobulinemia and marginal zone lymphoma, as well as under accelerated approval status for mantle cell lymphoma
EFFICACY:
- Open-label, multi-center, single-arm trial, n=42, patients with cGVHD after failure of first line corticosteroid therapy and requiring additional therapy
- Endpoint: Best Overall Response Rate (ORR) and Sustained Response Rate based on Investigator Assessment
- ORR: 28 (67%), Sustained Response Rate 20 (48%)
SAFETY:
- Serious side effects: Severe hemorrhage, infections, cytopenia, atrial fibrillation, hypertension, second primary malignancies, tumor lysis syndrome. May harm developing fetus or newborn
- Common side effects: Fatigue, bruising, diarrhea, thrombocytopenia, muscle spasms, stomatitis, nausea, hemorrhage, anemia and pneumonia

MAVYRET (glecaprevir and pibrentasvir) tablet
AbbVie Inc.
INDICATION:
- Treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A)
- Treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both
ADDRESSING UNMET NEED:
- 2.7 to 3.9 million people in US have chronic HCV
- First eight week treatment duration; provides a shorter treatment duration for many patients
- Treatment option for certain patients with genotype 1 infection, the most common HCV genotype in US
REG PATHWAY: NDA
- Required pediatric assessment
- Postmarketing commitments: Reports form ongoing studies, study in HCV
genotype 1, phenotypic effect of the NS3/4A or NS5A substitutions
MECHANISM OF ACTION: Fixed-dose combination of glecaprevir and pibrentasvir, which are direct-acting antiviral agents against HCV
EFFICACY:
- Several clinical trials, n=2,300 adults with genotype 1, 2, 3, 4, 5 or 6 HCV infection without cirrhosis or with mild cirrhosis, 12 or 16 weeks
- Serum HCV RNA values were measured during the clinical trials using the Roche COBAS AmpliPrep/COBAS Taqman HCV test
- 92-100% with no virus detected in blood 12 weeks after finishing treatment, suggesting that patients’ infection had been cured
SAFETY:
- Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected adult patients
- Not recommended in patients with moderate cirrhosis and contraindicated in patients with severe cirrhosis. It is also contraindicated in patients taking the drugs atazanavir and rifampin.
- Most common adverse reactions: Headache, fatigue and nausea
Image Credit: Celgene, Jazz, Janssen, AbbVie