FDA BRIEF: Week of June 12, 2017
FDA Science: Working at the Speed of Emerging Technologies
By: Luciana Borio, M.D., Acting Chief Scientist
Innovation is happening extraordinarily fast in biomedical sciences and at FDA
- FDA’s 11,000 scientists play essential role in advancing biomedical innovations
- Scientific research presented at Science Forum at FDA
- Research concentrates on developing knowledge to ensure medical products are safe and effective
2017 Science Forum topics
- Identification and Evaluation of New Biomarkers
- FDA Response to Urgent Public Health Needs
- Microbiome and Human Health
- Advanced Manufacturing and 3D Printing
- Omics Technologies at FDA
- Patient and Consumer Engagement and Communication
- Computational Modeling and Simulation at FDA
- Current Progress in Nanotechnology Research at FDA
Mobile communications also included
- Healthy Citizen @FDA to collaborate and communicate with citizens on public health outcomes
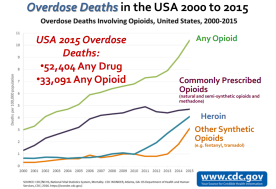
Everyone at FDA is committed to focusing on all aspects of the opioid epidemic
New policy steps
- Steering committee formation to examine regulatory and policy action
- Evaluate efforts to reduce number of new addiction cases
Abuse-deterrent opioid formulations and public health effect assessment
- Formulations intended to deter abuse
- However, effect in a real-world, meaningful decrease of opioid misuse/abuse unknown
- Public meeting on opioid medications with abuse-deterrent properties to discuss impact of these products in the real world
- Publicly available issues paper outlining existing regulatory and public health challenges

How Creative FDA Regulation Led to First-in-the-World Approval of a Cutting-Edge Heart Valve
By: Jeffrey Shuren, M.D., J.D., and Bram Zuckerman, M.D.
FDA was first to approve Sapien 3 valve interatin to treat high-risk patients with transcatheter valve replacement (TAVR)
- For high-risk patients with worn out aortic or mitral bioprosthetic valves; Sapien 3 slips into these valves – “valve-in-valve” option
- FDA Heart Valve Review Team streamlined FDA’s nonclinical testing expectations
- More consistent, predictable, and transparent about clinical study expectations
- Industry collaboration on creative clinical trial designs and use of other sources of clinical evidence
Use of real-world evidence for approval
- Based on Transcatheter Valve Therapy (TVT) Registry, partnership of American College of Cardiology and Society of Thoracic Surgeons
- Collection of clinical data on TAVR performance both on-label and off-label uses
- 100,000 TAVR patients since 2011 first approval; >600 patients for off-label, valve-in-valve uses
- Reliance on real-world evidence to evaluate the benefits and risks of off-label use
FDA working to broaden and improve the opportunities to leverage real-world evidence
- Establishment of National Evaluation System for health Technology (NEST)
- Integrate data from clinical registries, electronic health records, and medical billing claims
Image credits: FDA