FDA Approvals: SYMJEPI
FDA BRIEF: Week of June 12, 2017

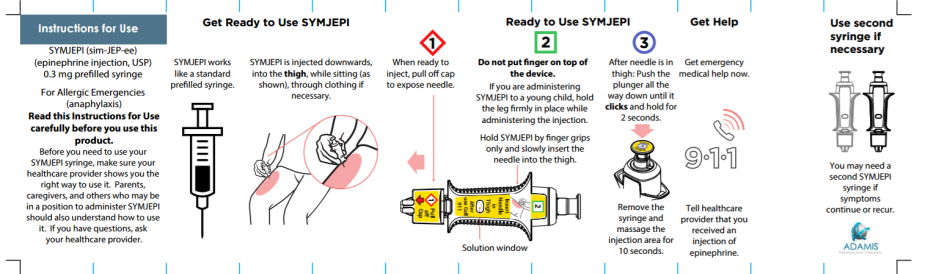
SYMJEPI (epinephrine injection)
Adamis Pharmaceuticals Corporation
INDICATION:
Emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which include bees, wasps, hornets, yellow jackets and fire ants) and biting insects (e.g., triatoma, mosquitoes), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media) and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis.
For immediate administration in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
Anaphylactic reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria or angioedema.
For immediate administration as emergency supportive therapy only and is not a
substitute for immediate medical care.
ADDRESSING UNMET NEED: New drug alternative to EpiPen
REG PATHWAY: NDA
- 505(b)(2) pathway
- Postmarketing Commitment: Fault Tree Analysis and quantification to support device reliability specification
MECHANISM OF ACTION: Acts on both alpha and beta-adrenergic receptors
EFFICACY & SAFETY: Established based on initial approval in 1939
Image credit: Adamis