FDA BRIEF: Week of December 19, 2016

RUBRACA (rucaparib) tablets, Clovis Oncology, Boulder, CO, USA
FoundationFocus CDxBRCA test, Foundation Medicine Inc. Cambridge, MA


INDICATION: Monotherapy for patients with deleterious BRCA mutation (germline and/or somatic) associated advanced ovarian cancer who have been treated with two or more chemotherapies.
Select patients for therapy based on an FDA-approved companion diagnostic for Rubraca
UNMET NEED:
- 22,280 women diagnosed with ovarian cancer ; 15-20% with BRCA gene mutation
- Targeted agents to treat cancers caused by specific mutations patient’s genes
- Additional treatment option for ovarian cancer patients with gene abnormalities
REG PATHWAY: NDA (drug) + approval of first next-generation sequencing (NGS)-based companion diagnostic
- Accelerated Approval, Breakthrough Therapy designation, Priority Review status, Orphan Drug designation
- Approved 2 mo. prior to PDUFA goal date
- Accelerated approval based on objective response rate and duration of response – continued approval contingent upon verification and description of clinical benefit in confirmatory trials
MECHANISM OF ACTION:
Rucaparib
- Poly (ADP-ribose) polymerase (PARP) enzyme inhibitor
- Enzymes play a role in DNA repair
- Increased rucaparib-induced cytotoxicity in tumor cell lines with BRCA1/2 deficiencies
CDxBRCA test : NGS based, detects alterations in BRCA1 and BRCA2 genes in the tumor tissue of ovarian cancer
EFFICACY:
- 2 multicenter, single-arm, open-label clinical trials (n=106), patients with advanced BRCA-mutant ovarian cancer, RUBRACA until disease progression or unacceptable toxicity
- Endpoint:. Objective response rate (ORR), duration of response (DOR) – by investigator, independent radiology review according to RECIST
- Objective Response Rate (95% CI) 54% (44, 64)
- Complete Response 9%
- Partial Response 45%
- Median DOR in months (95% CI) 9.2 (6.6 ,11.6)
SAFETY:
-
Most common adverse reactions: Nausea, fatigue (including asthenia), vomiting, anemia, abdominal pain, dysgeusia, constipation, decreased appetite, diarrhea, thrombocytopenia, and dyspnea.
-
Myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) reported
DEXCOM G5 Mobile Continuous Glucose Monitoring System
Dexcom, Inc. San Diego, CA, USA
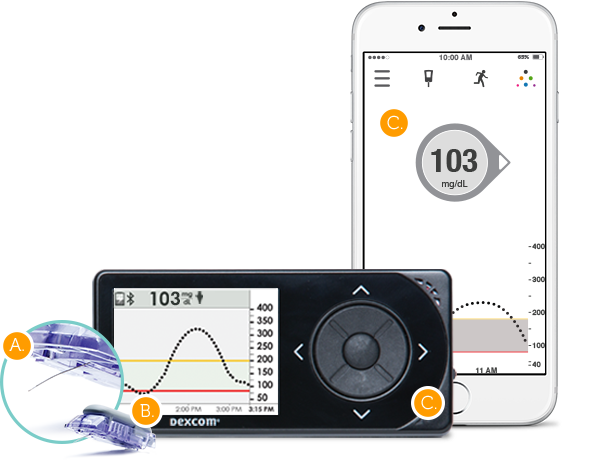
INDICATION FOR USE: Glucose monitoring system indicated for the management of diabetes in persons age 2 years and older. The Dexcom G5 is designed to replace fingerstick blood glucose testing for diabetes treatment decisions. Interpretation of the Dexcom G5 results should be based on the glucose trends and several sequential readings over time. The Dexcom G5 also aids in the detection of episodes of hyperglycemia and hypoglycemia, facilitating both acute and long-term therapy adjustments. The Dexcom G5 is intended for single patient use and requires a prescription.
REG PATHWAY: PMA Supplement
- To expand Indication for Use (non-adjunctive use to make treatment decisions)
DESCRIPTION:
- Sensor, transmitter, receiver, and mobile application
- Sensor: Inserted into subcutaneous tissue to generate electrical current proportional to the local glucose concentration
- Transmitter: Converts electrical current into glucose readings using algorithm; uses Bluetooth Low Energy (BLE) to communicate with Dexcom G5 receiver and Apple iOS device- receives blood glucose calibration and other user inputs
- Displays glucose reading and trends, alerts for readings outside of a target zone etc.
- Mobile application: User interface
EFFECTIVENESS & SAFETY:
- Clinical studies + discussion/recommendations of Clinical Chemistry and Toxicology Devices Panel + input provided by patients and caregivers on device experience
- 2 PMA clinical studies established trend accuracy, precision, calibration frequency , wear period, performance of alarms and alerts, number of readings displayed
- Possible adverse device effects: local infection, inflammation, pain or discomfort, bleeding at the glucose insertion site, bruising, itching, scarring or skin discoloration, hematoma, tape irritation, sensor or needle fracture during insertion, wear or removal.
AEROFORM Tissue Expander
AirXpanders, Palo Alto, CA, USA

INDICATION FOR USE: For soft tissue expansion in two-stage breast reconstruction following mastectomy and in the treatment of underdeveloped breasts and soft tissue deformities. A patient uses a dose controller to independently inflate the expander.
UNMET NEED:
- Currently used saline-filled tissue expanders requiring needle to pierce skin and inject saline into the expander
- Need for needlee-free option and for patients to have control over expansion, home use
REG PATHWAY: De Novo
DESCRIPTION:
- 2 main components: Expander and Controller
- Expander: Sterile implant with outer silicone shell; reservoir of compressed carbon dioxide
- Controller: Hand-held remote dosage controller
- Controller used to communicate to valve in reservoir to release carbon dioxide and gradually inflate the expander
- Controller pre-programmed to limit releasing a small amount of carbon dioxide once every three hours, up to a maximum of three times per day.
EFFECTIVENESS & SAFETY:
- Clinical trial, AeroForm expander (n=99) vs. saline expander (n=52)
- Endpoint: Breast tissue successfully expanded and exchanged to a breast implant
- 96.1% (AeroForm) vs. 98.8% (saline)
- Common adverse events: Necrosis, seroma, post-operative wound infection and procedural pain