 FDA News: Week of December 5 and 12, 2016
FDA News: Week of December 5 and 12, 2016
21st Century Cures Act: Making Progress on Shared Goals for Patients
By: Robert M. Califf, M.D., FDA Commissioner

President Obama signed into law the 21st Century Cures Act, on Dec 13th
- Builds on FDA’s ongoing efforts to advance medical product innovation, quick patient access, assurance of high quality evidence of safety and effectiveness
- Improves FDA’s ability to hire and retain scientific experts
Focus on:
- Incorporation of patient’s voice into FDA’s decision-making
- Modernizing and improving efficiency in clinical trial design
- Effective FDA engagement for expediting product development and application reviews
- New pathways for antibacterials/antifungals, regenerative medicine products
- Real world post market data for conducting more efficient research
- Healthcare economic information to payers and formulary committees
CDER PERFORMANCE


Novel New Drug Approvals
- Received 36 NME applications
- Approved 19 NMEs*, including 7 Orphan Drugs
Reasons for fewer NMEs compared to CY15
- Approval of 5 NMEs in CY15 with CY16 due dates
- Fewer NME actions in CY16
- Increased number of CR letters in CY16
Expedited Review
- Priority Review : 68%
- Breakthrough Therapy designation : 32%
- Fast Track designation : 37%
Drug Innovation
- Rare diseases : 37%
- First in class : 37%
- First approved in US : 84%
CDRH PERFORMANCE

- Guidance Documents: 58
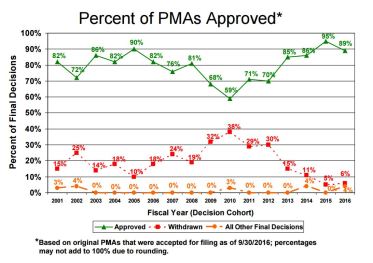
- PMA: 89% approved
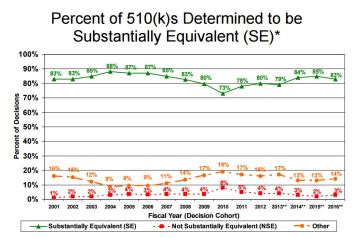
- 510(k): 83% Substantially Equivalent
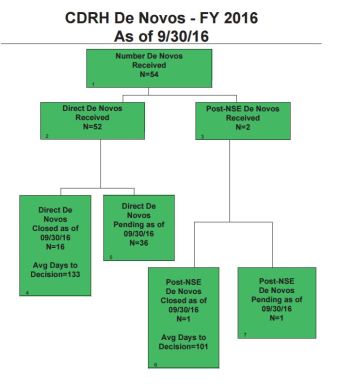
- Direct De Novo: 30%
- Pre-Submission Meetings: 939
Includes Division Level Data : DAGRID, DCD, DNPMD, DOD, DOED, DRGUD, DSD, DCTD, DIHD, DMD, DMGP, DRH
Faster Information from FDA Means Improved Drug Safety for Patients
Mary E. Kremzner, PharmD, MPH, CAPT, U.S. Public Health Service, Director, Division of Drug Information, CDER
 Easy, FAST, Up-to-Date drug safety information for health care professionals and patients
Easy, FAST, Up-to-Date drug safety information for health care professionals and patients
- Information on 18,000 drugs available at Drugs @ FDA website
- Drug Safety Labeling Changes Program posts real-time latest safety information
- Detailed prescribing information in product “labeling.”
- All databases easily searchable
The Mutual Reliance Initiative: A New Path for Pharmaceutical Inspections in Europe and Beyond
Dara Corrigan, J.D., Associate Commissioner for Global Regulatory Policy

- Concern: Rapid increase in imported drugs from nations with limited inspection resources e.g. China and India
- Solution: FDA partnering with EU to rely on each other’s inspections, avoid duplication, conduct more inspections
Initiative: Mutual Reliance Initiative (MRI)
- Launched in May 2014 -mutual recognition agreement
- “Brexit” has no impact on FDA’s relationship with UK counterparts at this time
- Key component covered in Transatlantic Trade and Investment Partnerships (T-TIP)