Webinar – Final Guidance on Medical Device Reporting for Manufacturers

SUMMARY
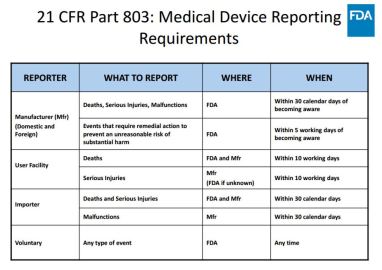
Reporting requirements for:
- Manufacturer
- Device user facilities
- Importers
Reporting of:
- Death
- Serious injury
- Malfunctions
Reporting Followups:
- Complete investigation of each event
- Develop & Implement reporting procedures
- Establish & Maintain reporting files
- Create system for expedited information access for follow-up/FDA inspection
SUMMARY SLIDE