FDA BRIEF: Week of October 17, 2016

LARTRUVO (Olaratumab) injection
Eli Lilly and Company, Indianapolis, Indiana, USA
INDICATION: In combination with doxorubicin, for the treatment of adult patients with soft tissue sarcoma (STS) with a histologic subtype for which an anthracycline-containing regimen is appropriate and which is not amenable to curative treatment with radiotherapy or surgery.
UNMET NEED:
- 12,310 new cases of STS and nearly 5,000 deaths in 2016
- New treatment option, added to doxorubicin, for the initial treatment of soft tissue sarcoma since doxorubicin’s approval more than 40 years ago
REG PATHWAY: Fast Track, Breakthrough Therapy Designation, Priority Review, Accelerated Approval, Orphan Drug Designation
- Post Approval Commitment: Randomized, controlled trial to verify and further describe the clinical benefit is STS
MECHANISM OF ACTION: Human IgG1 antibody that binds platelet-derived growth factor receptor alpha (PDGFR-α), expressed on cells of mesenchymal origin and detected on tumor cells.
EFFICACY:
- Open-label, randomized, active-controlled study (n=133), patients with soft tissue sarcoma not amenable to curative treatment with surgery or radiotherapy, LARTRUVO in combination with doxorubicin vs. doxorubicin
- Endpoints: Overall Survival (OS), Progression-Free Survival (PFS), Objective Response Rate (ORR) assessed by investigator and independent review using RECIST v1.1.
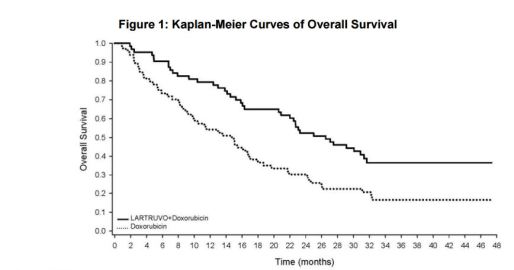
- Statistically significant improvement in OS: 26.5 mo. vs. 14.7 mo. (p<0.05)
- PFS: 8.2 mo. vs. 4.4 mo.
- ORR: 18.2 % vs. 7.5 %
SAFETY:
- Serious risks: Infusion-related reactions and embryo-fetal harm
- Most common side effects: Nausea, fatigue, low levels of white blood cells (neutropenia), musculoskeletal pain, inflammation of the mucous membranes (mucositis), hair loss (alopecia), vomiting, diarrhea, decreased appetite, abdominal pain, nerve damage (neuropathy) and headache.
BARRICOR Lithium Heparin Plasma Blood Collection Tubes (BD Barricor™ Tubes)
Becton, Dickinson and Company, USA

INDICATION FOR USE: Collect, separate, process, transport and store venous blood samples for use in chemistry determinations, therapeutic drug monitoring (TDM), and zinc testing in plasma for in vitro diagnostic use. It is used in settings where a venous blood sample is collected by a trained healthcare worker.
UNMET NEED:
- Reduce the amount of blood drawn from each patient for testing
- Reduce processing time in clinical labs.
REG. PATHWAY: 510(k) – Substantial Equivalence, CFR 862.1675 (Blood specimen collection devices), Class II, Product code: JKA
- Predicate device: 3 BD Vacutainer® Brand PST™ Plasma Separation Tube
DEVICE DESCRIPTION:
- Sterile (interior), single-use, evacuated blood collection tubes with mechanical separator (in place of gel), a low-zinc stopper and a plastic BD Hemogard™ color-coded lime green safety-engineered shield
- Interior spray coated with lithium heparin anticoagulan
- Novel separation technology which remains stable in its initial position, to enable the blood to be filled via current methods and subsequently creates a stable, robust barrier during processing
PERFORMANCE CHARACTERISTICS:
Analytical
- Precision/Reproducibility, Traceability, Stability, Expected values
Comparison to Comparator:
- 7 studies in healthy subjects and hospitalized patients
- Blood samples collected into the BD BarricorTM tubes (candidate device) and the comparator tubes (BD PSTTM (k945952) for chemistry and BD Serum tube (k960250) for TDM)
- Evaluations on selective common general chemistry analytes, immunology analytes, special chemistry analytes, cardiac markers, and therapeutic drug monitoring analytes on multiple instrument platforms
- 62 analytes (55 chemistry and 7 TDM) evaluated
- Comparable results between candidate tube and the comparator tubes.
TECENTRIQ (Atezolizumab) injection
Genentech, San Francisco, CA, USA.
INDICATION: Treatment of patients with metastatic non-small cell lung cancer (NSCLC) who have disease progression during or following platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving TECENTRIQ
REG PATHWAY: BLA, Added new indication
MECHANISM OF ACTION: Programmed death-ligand 1 (PD-L1) blocking antibody that previously received FDA accelerated approval for the treatment of locally advanced or metastatic urothelial carcinoma that has progressed after platinum-containing chemotherapy.
EFFICACY:
- 2 randomized, open-label clinical trials (n=1137) , patients with NSCLC, Compared atezolizumab vs docetaxel
- Primary endpoint: Overall Survival (OS)
- Median OS: 13.8 mo. vs. 9.6 mo, ( p=0.0004, Study 1), 12.6 mo. vs. 9.7 mo (Study 2)
SAFETY:
- Most common adverse reactions: Fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation
- Clinically significant immune-related adverse events: Pneumonitis, hepatitis, colitis, and thyroid disease.

- Regulation Name: Oral removable palatal space occupying device for weight management and/or weight loss Regulatory
- Classification: II
- Product Code: ONY
DEVICE DESCRIPTION:
- Oral removable palatal space occupying device
- Worn during meals to limit bite size, thereby reducing the amount of food that is consumed
- Recording sensors for monitoring patient use
