FDA BRIEF: Week of May 30, 2016


SCOPE
FDA policy on categorizing investigational device exemption (IDE) devices to assist CMS in determining coverage (reimbursement)
FDA INTERPREATION OF MEDICARE COVERAGE CATEGORIES A AND B
Category A: Experimental
- No PMA approval, 510(k) clearance or de novo request has been granted
- Proposed device has different characteristics compared to a legally marketed device
- Proposed device is being studied for a new indication or new intended use
Category B: Nonexperimental/Investigational
- No PMA approval, 510(k) clearance or de novo request has been granted
- Proposed device has similar characteristics compared to a legally marketed device
- Proposed device is being studied for a new indication or new intended use
CONSIDERATIONS FOR CHANGING FROM CATEGORY A TO B

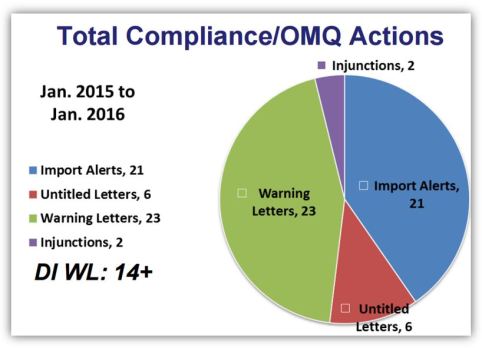
Inspecting for Data Integrity: From Manufacturing Floor to Quality Control Laboratories
US CFR REQUIREMENTS
- Backup data are exact and complete, and secure from alteration, inadvertent erasures, or loss
- Data stored to prevent deterioration or los
- Certain activities documented at the time of performance
- Laboratory controls be scientifically sound
- True copies or other accurate reproductions of the original records
- Complete information, complete data derived from all tests, complete record of all data, and complete records of all tests performed.
INSPECTION TRENDS
- Responsibilities andapplicable procedures not in writing or fully followed
- Laboratory controls do not include scientifically sound and appropriate specifications, standards, sampling plans, and test procedures
- Failure to thoroughly review any unexplained discrepancy or the failure of a batch
- Procedures designed to prevent microbiological contamination not established
- No written procedures for identity, strength, quality, and purity
- Deficient aseptic processing areas
- Routine calibration, inspection and checking of equipment not performed