FDA BRIEF: Week of May 9, 2016

IMAGE READY MR CONDITIONAL PACING SYSTEM
Boston Scientific Corporation, St. Paul, MINNESOTA, USA

INDICATION FOR USE:
Treatment of the following conditions:
- Symptomatic paroxysmal or permanent second- or third-degree AV block, Symptomatic bilateral bundle branch block
- Symptomatic paroxysmal or transient sinus node dysfunction with or without associated AV conduction disorders
- Bradycardia-tachycardia syndrome
- Neurovascular (vaso-vagal) syndromes
REG PATHWAY: PMA
DEVICE DESCRIPTION:
- ImageReady System created specifically for use with MRI scans
- Includes ACCOLADE™ MRI and ESSENTIO™ MRI pacemakers, as well as the new INGEVITY™ MRI pacing leads
- Pacemaker design has minimized use of ferromagnetic materials, which can interact with the fields generated during a typical MRI scan, and circuits have been designed to tolerate voltages that may be induced during scans
- Lead wire designed to reduce absorption of energy from MR Fields, thus minimizing subsequent heating
- System for full body scan, with no thoracic exclusion zone
EFFECTIVENESS:
INGEVITY study: Prospective, non-randomized study, n =1,036 patients, patients with a single or dual chamber pacemaker
- Primary Endpoints (3 mo. post-implant): Pacing Threshold at 0.5 ms pulse, Sensed Amplitude, Pacing Impedance
- Met the pre-specified effectiveness endpoints at 3 months of follow-up
SAMURAI study: Prospective, global,open-label, two-group randomized to receive a protocol-required MRI scan (MRI Group) or not receive a scan (Control Group).
- Primary Endpoints (pre and 1-mo. post MRI): Change in Pacing Threshold (at 0.5 ms pulse width), Change in Sensed Amplitude
- Performed acceptably while used in the MRI environment. The clinical study met the pre-specified effectiveness endpoints at the MRI + 1 month (3 months post-implant) follow-up.
SAFETY:
- Suitable for longterm implant
- Adverse event rates comparable to other marketed pace/sense leads
IMPELLA 2.5 SYSTEM
ABIOMED, Inc., Danvers, MASSACHUSETTS, USA
INDICATION FOR USE:
Temporary ventricular support devices intended for:
- short term use (≤ 4 days for the Impella 2.5)
- treatment of ongoing cardiogenic shock that occurs immediately (< 48 hours) following acute myocardial infarction or open heart surgery
- intent is to reduce ventricular work and to provide the circulatory support necessary to allow heart recovery and early assessment of residual myocardial function
REG. PATHWAY: Supplemental PMA to expand indication for use to include cardiogenic shock. Original PMA approved March 2015. Classification: OZD
DEVICE DESCRIPTION:
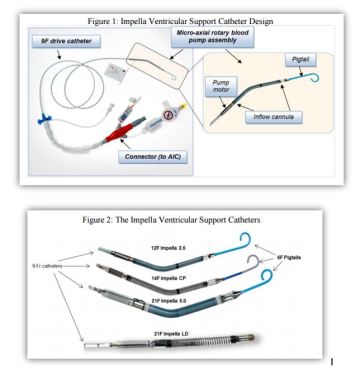
- Catheter consist of micro-axial rotary blood pump mounted on a 9F drive catheter, connected to external controller -Automatic Impella Controller (AIC).
- 4 different Impella support Catheters to accommodate range of cardiac flow requirements and implant techniques
- Catheter placed with cannula inflow located in left ventricle and outflow located in ascending aorta
- AIC generates signals power drive motor of Catheter and provides user interface
- AIC also incorporates disposable Impella Purge Cassette system fluid pressure barrier
- AIC qualified for use for patient transport by trained healthcare professionals
EFFECTIVENESS:
- Prospective, singlearm study, n=18 (RECOVER I) (+ Impella Registry, comparative analysis vs approved surgical Ventricular Assist Device, VAD, literature review)
- Primary Endpoint: Survival to:
- Recovery defined as 30-day survival post-explant or hospital discharge
- Bridge-to-other-therapy
- Survival met in 88% of the cases
- Consistent and reproducible hemodynamic support
- Rapid wean of patients off of inotropes and pressors

SAFETY:
- 2 serious adverse events : hemolysis, infection; both resolved
- Overall profile favorable vs approved VAD.
FLUENCY PLUS ENDOVASCULAR STENT GRAFT
Bard Peripheral Vascular, Inc. Tempe, ARIZONA, USA
INDICATION FOR USE: Treatment of in-stent restenosis in the venous outflow of hemodialysis patients dialyzing by either an arteriovenous (AV) fistula or AV graft and for the treatment of stenosis in the venous outflow of hemodialysis patients dialyzing by an AV graft.
REG PATHWAY : Supplement PMA to add stenosis treatment. Original PMA approved June 2014
DEVICE DESCRIPTION:
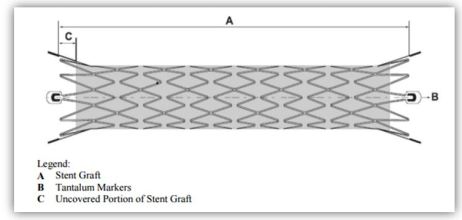
- Flexible, self-expanding endoprosthesis comprised of expanded polytetrafluoroethylene (ePTFE) encapsulating a Nitinol stent framework
- Nitinol, an alloy, can be processed to assume a pre-defined final configuration upon exposure to body temperature
- Four radiopaque tantalum markers on each end facilitating stent graft placement
- Nitinol stent encapsulated with ePTFE except graft ends
EFFECTIVENESS:
- Randomized, prospective, multi-center clinical trial (RESCUE); also leveraged data from original PMA
- Primary endpoint: Access Circuit Primary Patency (ACPP) at 6 m0.
- ACPP rate significantly higher (p<0.001) in FLUENCY arm vs. control
- Secondary Endpoint: Post-Intervention Lesion Patency (PLP) at 6 mo.
- PLP significantly higher (p<0.001) in the FLUENCY arm vs. control
SAFETY:
- Similar to original PMA; included in Labeling
TRIDYNE VASCULAR SEALANT
Neomend, Inc. Irvine, CALIFORNIA, USA

INDICATION FOR USE: use in aortic surgery when adjunctive measures to achieve hemostasis are required by mechanically sealing areas of leakage
REG PATHWAY: PMA, Code NBE
DEVICE DESCRIPTION:
- Single-use, formed by mixing (1) Human Serum Albumin (HSA) and (2) synthetic crosslinking component of polyethylene glycol (PEG)
- Delivery system used to deliver hydrogel at intended treatment site
EFFECTIVENESS:
- Prospective, multicenter, randomized, single-blind (subject), superiority clinical study, n=156, TRIDYNE vs. Control for intraoperative bleeding control after thoracic surgery
- Primary endpoint: Time to hemostasis (TTH)
- TTH : Significantly lower with TRIDYNE, 124.3 sec vs 377.8 sec, p< 0.0001
- Higher proportion of patients in TRIDYNE arm achieved immediate hemostasis, hemostasis within 5 min, hemostasis within 10 min
SAFETY:
- Deaths: 2 in TRIDYNE vs. 1 in Control. Not considered device related
- No major differences in adverse events between groups