FDA BRIEF: WEEK OF MAY 2, 2016


NUPLAZID (pimavanserin) tablets
Acadia Pharmaceuticals, San Diego, California, USA
INDICATION: Treatment of hallucinations and delusions associated with Parkinson’s disease psychosis
UNMET NEED:
- ~ 50,000 Americans diagnosed with Parkinson’s disease each year
- Hallucinations or delusions can occur ~in as many as 50 percent of patients
- Can be profoundly disturbing and disabling
REG PATHWAY: Breakthrough therapy designation, Priority Review
MECHANISM OF ACTION: Exact mechanism unknown. Could be mediated through combination of inverse agonist and antagonist activity at serotonin 5-HT2A and 5-HT2C receptors.
EFFICACY:
- Single 6-week, randomized, placebo-controlled, parallel-group study. (n=199)
- Primary Endpoint: Parkinson’s Disease -adapted Scale for the Assessment of Positive Symptoms (SAPS-PD), at 6 weeks; measured by central, independent, and blinded raters
- Superior to placebo in decreasing frequency and/or severity of hallucinations and delusions
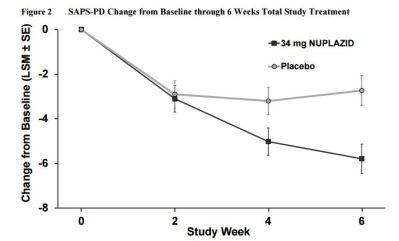
SAFETY:
- Boxed Warning: An increased risk of death associated with the use (same as other atypical antipsychotic drugs)
- Most common side effects: Swelling, usually of the ankles, legs, and feet due to the accumulation of excessive fluid in the tissue; nausea; and abnormal state of mind


B·R·A·H·M·S PCT sensitive KRYPTOR
B·R·A·H·M·S GmbH, part of Thermo Fisher Scientific, Berlin, GERMANY
INDICATIONS FOR USE
- Immunofluorescent assay using Time-Resolved Amplified Cryptate Emission (TRACE) technology to determine the concentration of PCT (procalcitonin) in human serum and EDTA or heparin plasma
- Intended for use in conjunction with other laboratory findings and clinical assessments to aid in the risk assessment of critically ill patients on their first day of Intensive Care Unit (ICU) admission for progression to severe sepsis and septic shock
- Also to determine the change in PCT level over time as an aid in assessing the cumulative 28-day risk of all cause mortality
REG PATHWAY: De Novo, Class II, Code PMT
DEVICE DESCRIPTION:
- pipetting module
- reading module
- external bottles for fluidic system
- external PC
- handheld barcode scanner
- software
EFFECTIVENESS:
- Prediction of 28-d cumulative all-cause mortality in patients with severe sepsis/septic shock (n=858). PCT levels measured on Days 0, 1, 4
- Percent change in PCT level over Day 4 significantly associated with 28-d cumulative mortality;
- Stratification by hospital location gave significant correlation
- Mortality Risk and Prognostic Accuracy base don initial PCT levels determined
RISKS (MITIGATION): Improper patient management
- False positives/negatives (Special Controls including detailed Instructions for Use, Labeling)
- Incorrect interpretation (Special Controls including Labeling, website with ‘PCT change calculator’)
- Manual Calculation error of final results (Labeling)