FDA BRIEF: Week of April 25, 2016


CABOMETYX (Cabozantinib)
Exelixis Inc., South San Francisco, California, USA
INDICATION: Treatment of patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy
REG PATHWAY: Breakthrough Therapy Designation, Fast Track, and Priority Review; approved prior to the Priority Review deadline of June 22, 2016
MECHANISM OF ACTION: Inhibits the tyrosine kinase activity of receptors involved in both normal cellular function and pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, drug resistance, and maintenance of the tumor microenvironment.
EFFICACY:
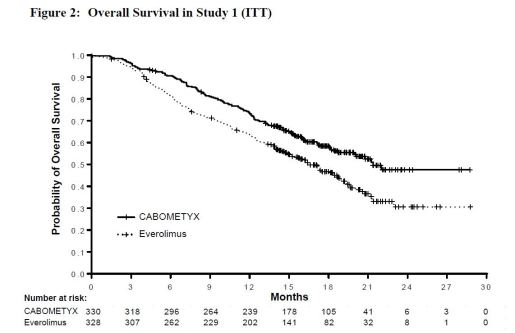
- Single pivotal, randomized (1:1, (N=658)), open-label, multicenter study, CABOMETYX vs. everolimus, in patients with advanced RCC woith 1 prior therapy
- Primary outcome: Progression-free survival (PFS) : 7.4 mo. vs. 3.8 mo. (p<0.0001)
- Overall Survival : 21.4 mo. vs. 16.5 mo. (p<0.0001)
SAFETY:
- Most common serious adverse events: Abdominal pain, pleural effusion, diarrhea, nausea
-
Most common adverse reactions: Diarrhea, fatigue, nausea, decreased appetite, palmar-plantar erythrodysesthesia syndrome, hypertension, vomiting, weight decreased, constipation
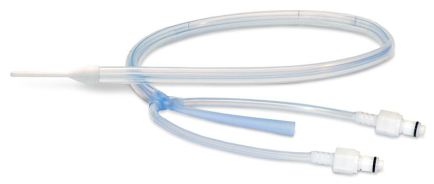
ESOPHAGEAL COOLING DEVICE
Advanced Cooling Therapy, Inc., Chicago, Illinois, USA
INDICATION: Thermal regulating device, intended to:
- connect to a Gaymar Medi-Therm III Conductive Hyper/Hypothermia System to control patient temperature
- provide gastric decompression and suctioning
REG PATHWAY: De Novo, Class II
DEVICE DESCRIPTION:
- Silicone tube with three lumens that is placed in the esophagus
- Control’s patient’s temperature, while simultaneously maintaining access to the stomach to allow gastric decompression and drainage
- Modulation and control of patient temperature achieved by connecting to external heat exchanger and circulating temperature controlled fluid
CLINICAL DATA:
- 16 patients, from centers outside US where device is approved
- Data summaries of therapeutic cooling, maintenance of target temperature, eventual rewarming
- Cooled and eventually rewarmed patients resuscitated from cardiac arrest
- Duration of temperature management: 24-36 hrs
- No evaluation whether hypothermia improved cardiac arrest outcomes