FDA Brief: Week of Mar 21, 2016

ANTHIM (obiltoxaximab) injection
Elusys Therapeutics, New Jersey, USA
Indication: In adult and pediatric patients
- For treatment of inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs
- For prophylaxis of inhalational anthrax due to B. anthracis when alternative therapies are not available or not appropriate [see Indications and Usage
Unmet Need:
- Rare disease caused by breathing spores of bacterium Bacillus anthracis
- Bacterial toxins produce massive and irreversible tissue injury and death
- Potential bioterrorism threat
Reg Strategy: BLA, approved under the FDA’s Animal Rule, Developed in in conjunction with the U.S. DHHS Biomedical Advanced Research and Development Authority (BARDA)
Mechanism of Action: Monoclonal antibody that neutralizes toxins produced by B. anthracis.
Efficacy: Rabbits (2 studies) and cynomolgus macaque (2 studies) as it is not feasible or ethical for human studies; with systemic anthrax; placebo controlled
- Survival : Significant improvement (p<0.01-0.001) in survival relative to placebo
- Survial (in combination with antibiotics) : Further improvement
- Survival (prophylaxis treatment): 100%
Safety: 320 healthy human volunteers.
- Boxed Warning: Hypersensitivity, Anaphylaxis
- Most frequently reported side effects: Headache, itching (pruritus), upper respiratory tract infections, cough, nasal congestion, hives, and bruising, swelling and pain at the infusion site.
TALTZ (ixekizumab) injection
Eli Lilly, Indiana, USA
Indication: Treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Unmet Need:
- Autoimmune disorder, with a family history, between ages of 15 and 35
- Thick, red skin with flaky, silver-white scales
- Need for treatment option to relieve the skin irritation and discomfort
Reg. Strategy: BLA, Standard review
Mechanism of Action: Humanized immunoglobulin G subclass 4 monoclonal antibody (mAb) Antibody binds to protein (interleukin -17A) that causes inflammation.
Efficacy: 3 multicenter, randomized, double-blind, placebo-controlled trials, n= 3866, 12 weeks. In 2 trials, subjects also randomized to etanercept for 12 weeks.
- Greater clinical response than placebo in the 2 co-primary endpoints
- Psoriasis Area and Severity Index (PASI) 75% reduction:
- Static Physician Global Assessment (sPGA) 2-pt improvement
Safety:
- Medication Guide on greater risk of an infection
- Serious allergic reactions and inflammatory bowel disease
- Most common side effects: Upper respiratory infections, injection site reactions and fungal (tinea) infections.
CINQAIR (reslizumab) infusion
Teva, Pennsylvania, USA
Indication: Add-on maintenance treatment of patients with severe asthma aged 18 years and older with an eosinophilic phenotype
Unmet Need:
- > 22 million in U.S. have asthma, > 400,000 asthma-related hospitalizations/yr
- Need for another treatment option
Reg Strategy: BLA, Standard review
Mechanism of Action: Humanized interleukin-5 antagonist monoclonal antibody produced by recombinant DNA technology in murine myeloma cells. Reduces blood eosinophils that contribute to asthma.
Efficacy: 4 randomized, double-blind, placebo-controlled studies (n=981), 52 weeks, 12 years of age and older
- Frequency of asthma exacerbations: Significant reduction
- Time to first attack: Significantly longer
- Improvement in Lung Function (FEV1): Improved

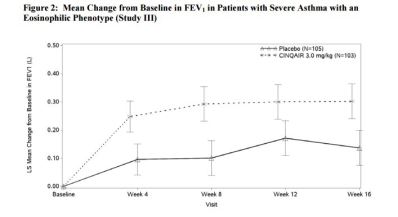
Safety:
- Can cause serious side effects including allergic (hypersensitivity) reactions
- Most common side effects: Anaphylaxis, cancer, muscle pain
Xalkori (crizotinib) capsules
Pfizer, New York, USA
Indication: Treatment of patients with metastatic non small-cell lung cancer (NSCLC) whose tumors are ROS1-positive (previously approved for NSCLC with anaplastic lymphoma kinase (ALK)-positive tumors)
Unmet need:
- 221,200 new diagnoses and 158,040 deaths in US in 2015
- Difficult to treat, different mutations, some of which are rare
- Need for treatment option for rare and difficult to treat ROS-1 gene mutation
Reg. Strategy: SNDA, Breakthrough Therapy designation, Priority Review, Orphan Drug designation
Efficacy: Single, multicenter, single-arm study (n=50), histologically-confirmed advanced NSCLC with a ROS1 rearrangement
- Objective Response Rate: 66%
- Duration of Response: 18.3 mo.
Safety:
- Serious side effects: Liver problems, life-threatening or fatal lung inflammation, abnormal heartbeats, vision loss
- Most common side effects: Vision disorders, nausea, diarrhea, vomiting, swelling (edema), constipation, liver problems (elevated transaminases), fatigue, decreased appetite, upper respiratory infection, and dizziness and numbness or tingling in the hands or feet