FDA Webinar Debrief
February 19, 2016

Presenters
CDRH, Human Factors Team
- Shannon Hoste, M.S., Human Factors Pre-Market Evaluation Team Member
- Xin Feng, Ph.D., Human Factors Pre-Market Evaluation Team Member
- Hanniebey Wiyor, Ph.D., Human Factors Pre-Market Evaluation Team Member
Presentation Overview
- Relevant regulations and standards
- FDA’s Human Factors guidance
- List of highest priority devices for human factors review – draft guidance
Human Factors/Usability Engineering
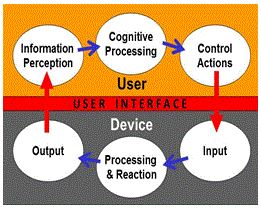
Focuses on interactions between people and devices. The critical element is the device user interface, depicted as the red zone in Figure below
.
FDA 2016 Guidances
Clarify expectations around when to submit a HF report with a premarket submission


Summarized previously in this blog
Key Takehomes
- Human Factors testing is a part of a robust design control subsystem
- Submit data in premarket submissions if risk analysis indicates user error could result in serious harm
- Consult FDA early using Q-Sub process to discuss and align on development plan and labeling implications