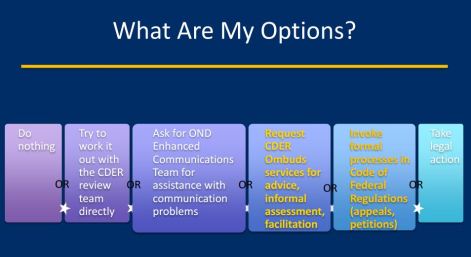
FDA Formal Dispute Resolution

WHAT: Formal Review of scientific and/or medical disputes. Related to applications for user fee products. To quickly and impartially investigate complaints and resolve disputes between CDER and CDER-regulated industry, health care practitioners, and consumers by offering an informal, confidential, and neutral environment
HOW: Process codified in Code of Federal Regulations (CFR) and CDER/CBER Guidance for Industry
WHO: FDA contacts:
- CDER Formal Dispute Resolution Project Manager
- Ombudsman