FDA Brief: Week of Feb 15, 2016

BRIVIACT® (brivaracetam) tablets
UCB, Inc. Smyrna, Georgia
Indication : Add-on treatment to other medications to treat partial onset seizures in patients age 16 years and older with epilepsy.
Unmet Need:
- Approximately 5.1 million people in the United States have a history of epilepsy and approximately 2.9 million people in the United States have active epilepsy
- Brain disorder that causes people to have recurring seizures
- Need for new treatment option
Reg Pathway: NDA, standard review
Mechanism of Action: Binding to the ubiquitous synaptic vesicle glycoprotein 2A
Efficacy:
- 3 randomized, double-blind, fixed-dose, multi-center studies (n=1,550); 8-week baseline period followed by a 12-week treatment period, patients with uncontrolled partial onset seizures despite concomitant antepileptic therapy; Briviact vs Placebo
- Effective in reducing the frequency of seizures.with Briviact vs placebo
Safety:
- Serious risks: Suicide thoughts / attempts, depression, aggression, panic attacks, allergic reaction
- Must be dispensed with Medication Guide
- Most common side effects: Drowsiness, dizziness, fatigue, nausea and vomiting

IBRANCE® (palbociclib) capsules
Pfizer, Inc., NY
Indication: Treatment of HR-positive, HER2-negative advanced or metastatic breast cancer in combination with:
- letrozole as initial endocrine based therapy in postmenopausal women, or
- fulvestrant in women with disease progression following endocrine therapy.
Reg Pathway:
- Accelerated approval in 2015 for combination treatment with letrozole for the treatment of HR-positive, HER2-negative advanced breast cancer as initial endocrine based therapy in postmenopausal women
- This approval based on study showing profression free survival (PFS); Prior Approval sNDA, , Priority Review, Breakthrough designation
Efficacy:
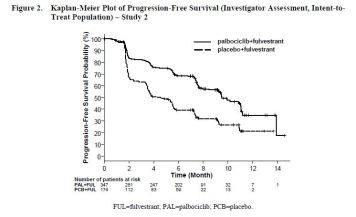
- Single international, randomized, double-blind, parallel group, multicenter study (n=521); IBRANCE plus fulvestrant vs. placebo plus fulvestrant (n=521; women with HR-positive, HER2-negative advanced breast cancer
- Major efficacy outcome: Investigator-assessed PFS evaluated according to RECIST 1.1: 41.8% vs 65.5% , p p<0.0001
- Median PFS was 9.5 versus 4.6 months
Safety:
- Most common adverse reactions: Neutropenia, leukopenia, infections, fatigue, nausea, anemia, stomatitis, headache, diarrhea, thrombocytopenia, constipation, vomiting, alopecia, rash, decreased appetite, and pyrexia