
 EMPLICITI (elotuzumab) for injection
EMPLICITI (elotuzumab) for injection
Indication: In combination with lenalidomide (Revlimid) and dexamethasone for the treatment of patients with multiple myeloma who have received one to three prior therapies
Unmet need:
- Multiple myeloma form of blood cancer in bone marrow
- 26,850 new cases and 11,240 related deaths in the US in 2015
- Need to therapies to provide additional benefit vs currently approved
Reg Pathway: BLA, Breakthrough Designation, Priority Review, Orphan Drug Designation
Mechanism of Action: Humanized IgG1 monoclonal antibody targeting SLAMF7 (Signaling Lymphocytic Activation Molecule Family member 7) expressed on myeloma cells and Natural Killer cells to mediate the killing of myeloma cells
Efficacy:
- Randomized open label (n=646); Empliciti + lenalidomide and dexamethasone vs . lenalidomide and dexamethasone
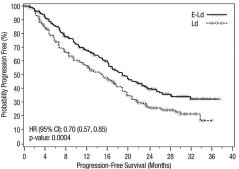
- Progression-Free Survival : Hazard Ratio (95% CI) :0.70 [0.57, 0.85], p = 0.0004
- Overall Response Rate : 252 vs.213, p = 0.0002
- 1-and 2-year PFS rates : 68% and 41% vs. 57% and 27%, respectively
Safety:
- Most common side effects: Fatigue, diarrhea, fever (pyrexia), constipation, cough, nerve damage resulting in weakness or numbness in the hands and feet (peripheral neuropathy), infection of the nose and throat (nasopharyngitis), upper respiratory tract infection, decreased appetite and pneumonia
Fluad (inactivated influenza vaccine containing adjuvant)
Indication: Aactive immunization against influenza disease caused by influenza virus subtypes A and type B contained in the vaccine; approved for use in persons 65 years of age and older
Unmet Need:
- 80 – 90 % of seasonal influenza-related deaths & 50 – 70 % of seasonal influenza-related hospitalizations occur in 65 years of age and older.
- Need for immunizing in this age group; to decrease disease burden as well as influenza-related hospitalizations and deaths
Reg Pathway: BLA, Accelerated Approval
Mechanism of Action: Inactivated trivalent influenza vaccine, containing hemagglutinin of influenza virus strains (two subtypes A and one type B), representing the viruses likely to be circulating in the US in winter
Efficacy: Approval is based on the immune response; data demonstrating a decrease in influenza disease after vaccination not available
- Single study (n= 7,082) Fluad vs Agriflu (unadjuvanted vaccine)
- Primary immunogenicity analyses:Non-inferiority for all three vaccine strains for antibody levels
Safety:
- No safety concerns
- Most common adverse events : Injection site pain and tenderness, muscle aches, headache and fatigue
 Portrazza (necitumumab injection)
Portrazza (necitumumab injection)
Indication: In combination with gemcitabine and cisplatin, for first-line treatment of patients with metastatic squamous non-small cell lung cancer
Unmet need:
- Lung cancer leading cause of cancer death in US
- 221,200 new diagnoses and 158,040 deaths in 2015
- Lung cancer tumors varied, so need tailored treatment options to extend survival
Reg Pathway: BLA, Standard Review
Mechanism of Action : Recombinant human lgG1 monoclonal antibody that binds to human epidermal growth factor receptor (EGFR) commonly found in lung cancer and blocks malignant progression, induction of angiogenesis, and inhibition of apoptosis.
Efficacy:
- Multicenter, randomized, open-label (n= 1,093(, Portrazza + chemotherapy (gem + cis) vs chemotherapy alone
- Overall Survival (median) : 11.5 mo. vs 9.9 mo. (p = 0.01)
- Progression Free Survival (median) : 5.7 mo. vs 5.5 mo. (p=0.02_
- No difference in Overall Response Rate

Safety:
- Most common side effects: Skin rash and magnesium deficiency (hypomagnesemia), which can cause muscular weakness, seizure, irregular heartbeats and can be fatal
- Boxed Warning: Serious risks of cardiac arrest and sudden death, as well as hypomagnesemia.
 Opdivo (nivolumab injection)
Opdivo (nivolumab injection)
Indication : Treat advanced (metastatic) renal cell carcinoma who have received prior anti-angiogenic therapy
Unmet need:
- Renal cell carcinoma is the most common form of kidney cancer
- 61,560 new cases and 14,080 deaths from kidney and renal pelvis cancer in the US in 2015 .
- Need for therapy to extend survival ( Torisel, approve din 2007, only agent to extend survival)
Reg Pathway : Supplemental BLA, Breakthrough Therapy Designation, Fast Track Designation, Priority Review
Mechanism of Action: Human immunoglobulin G4 (IgG4) monoclonal antibody that binds to the PD-1 receptor,releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response.
Efficacy:
- Single open-label randomized (n=821), Opdivo vs everolimus
- Overall Survival (median) : Median 25.0 mo. vs 19.6 mo. (p = 0.0018)
- Complete or partial tumor shrinkage : 21.5 % (for 23 mo.) vs 3.9 % (for 13.7 mo)

Safety:
- Most common side effects : Abnormal weakness or lack of energy (asthenic conditions), cough, nausea, rash, difficulty breathing (dyspnea), diarrhea, constipation, decreased appetite, back pain and joint pain (arthralgia).
- Serious side effects : “immune-mediated side effects” involve healthy organs, including the lung, colon, liver, kidneys, hormone-producing glands and the brain.
 BioThrax (Anthrax Vaccine Adsorbed)
BioThrax (Anthrax Vaccine Adsorbed)
Indication : Active immunization for the prevention of disease caused by Bacillus anthracis in persons 18 through 65 years of age.
Unmet Need:
- Anthrax disease, esp.inhalation form, is often fatal
- Need for vaccine to prevent disease after exposure to anthrax spores (Post Exposure prophylaxis – PEP)
Reg Pathway:Supplemental BLA, FIRST Approval based on Animal Rule.; allows animal efficacy data to be used as a basis for approval when human efficacy studies are not ethical or feasible.
10-year collaborative effort between Emergent, Biomedical Advanced Research and Development Authority (BARDA), National Institute of Allergy and Infectious Diseases. A 2010 pre-Phase 3 Vaccines and Related Biological Products Advisory Committee meeting confirmed the regulatory pathway
Mechanism of Action : Induces antibodies for neutralizing the activities of the cytotoxic lethal toxin and edema toxin of Bacillus anthracis
Efficacy :
Animal Models : To derive protective antibody thresholds to bridge animal efficacy and human immunogenicity data and predict efficacy in humans.
- Pivotal efficacy animal studies in rabbits and nonhuman primates: Model developed for 70% probability of survival
- 70-100% survival in animals receiving both antimicrobial treatment and vaccination vs antimicrobial treatment only
Human Studies : Safety and antibody responses
- 200 healthy adults in three doses at zero, two, and four weeks; antibody responses correlated to a 70 percent probability of survival observed in animal models.
Safety : Localized adverse events reported as tenderness, pain, swelling, and redness at the injection site, as well as limited movement of the injected arm. The most common systemic adverse reactions were muscle aches, headache, and fatigue

AngioJet™ ZelanteDVT™ Thrombectomy Catheter
510K Clearance, Boston Scientific
- Use with the AngioJet Ultra Console to break apart and remove thrombus, including deep vein thrombus (DVT), from:
- Iliofemoral and lower extremity veins ≥ 6.0 mm in diameter
- Upper extremity peripheral veins ≥ 6.0 mm in diameter.
- Infusion of physician specified fluids, including thrombolytic agents, into the peripheral vascular system.
- NOT been evaluated for pulmonary embolism (PE)
 Algovita Spinal Cord Stimulation (SCS) System
Algovita Spinal Cord Stimulation (SCS) System
510K Clearance, Nuvectra
- Aid in the management of chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with failed back surgery syndrome, intractable low back pain, and leg pain.
- 24-channel implantable pulse generators (IPGs)
- Wireless clinician programmer with computer-assisted stimulation programming to assist with effective and efficient localization, targeting and fine-tuning of pain coverage.
SilverCoat Silicone Foley Catheter
510K Clearance, Covalon Technology
- Greater bacterial kill than two of the leading antimicrobial catheters
- Minimizes biofilm formation and bacterial adherence
- Kills pathogens most associated with CAUTI (Catheter Associated Urinary Tract Infections)
IQQA-Guide, an intra-operative 3D navigation system for thoracic, abdominal, and pelvic surgery
510K Clearance, EDDA Technology
- Patient-specific anatomic models extracted from multi-modality images for 3D navigation during surgery
- Surgeon can “see through” organ surfaces; may reduce use of intra-operative CT scans during imaging-guided surgical procedures
FDA Drug Safety Communication:
WARNING: SGLT2 inhibitors for diabetes can cause too much acid in the blood and serious urinary tract infections
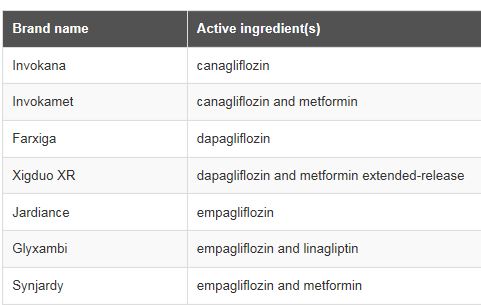
FDA DATA ANALYSIS and COMMUNICATIONS:
- March 2013 – May 2015: 73 cases of ketoacidosis with hospitalization, 19 cases of life-threatening blood infections (urosepsis) and kidney infections (pyelonephritis)
- May 2015 : Drug Safety Communication alert; continue to evaluate
- Now : Patients should stop taking their SGLT2 inhibitor and seek medical attention immediately if they have any symptoms of ketoacidosis. Health care professionals should assess for ketoacidosis and urinary tract infections in patients. Label updated to include WARNING

